Library
Browse resources published by our research team.
In addition to full texts of our peer-reviewed articles, our library includes research digests that break down our peer-reviewed articles; in-depth reports that thoroughly examine a topic; commentaries that explain the significance of particular issues in wild animal welfare science; and short communications that briefly survey a field or topic.
Wild Animal Initiative adheres to Open Science TOP Guidelines. Read more here.
Considering affective state as a central component of the response of animals to environmental changes
Beaulieu, M. (2026). Considering affective state as a central component of the response of animals to environmental changes. Biology Letters, https://doi.org/10.1098/rsbl.2025.0424
Authored by Wild Animal Initiative Research Manager Michaël Beaulieu, this paper was published in the January 2026 issue of Biology Letters.
Abstract
Current environmental changes are often considered as negatively impacting the affective state of animals. Yet, the interplay between environmental conditions and affective state should rather be viewed as a reciprocal and dynamic relationship, as variation in affective state likely determines how animals decide to respond to environmental changes. Here, I illustrate how affective states may contribute to determining how animals respond to environmental changes through phenotypic plasticity, environmental modification or dispersal. A condition for future studies to examine this hypothesis will be to consider the response of animals in parallel with valid indicators of affective state reflecting different affective dimensions (valence, arousal) over different timescales. Moreover, considering affective state as a central component of the response of animals to environmental changes implies that a condition to realistically investigate this response is to provide animals with the freedom to decide between options associated with different affective values.
Bye-bye bycatch: a remotely closing trap for targeted songbird capture
Bonnie Flint, Ben Vernasco
Flint, B. F., & Vernasco, B. J. (2025). Bye-bye bycatch: a remotely closing trap for targeted songbird capture. Journal of Field Ornithology, https://doi.org/10.5751/JFO-00744-960408
Authored by Wild Animal Initiative’s Researcher and Education Specialist, Bonnie Flint, this paper was published in the December 2025 issue of the Journal of Field Ornithology.
About the paper
This remotely closing bird trap was designed as part of our house sparrow research project, which aims to validate indicators of welfare in house sparrows and examine how varying environmental conditions affect their welfare.
Capturing and marking animals is often an essential component of studying wild populations, but the process of capturing and marking can have detrimental effects on captured birds. For instance, research projects are often focused on one or a few species, yet many other, non-target species can be inadvertently captured. Bycatch (non-target animals that are captured during the process of capturing target animals) is a frequent problem in ornithological research projects using non-selective capture methods such as mist nets, Potter traps, or other passive, baited traps. These non-target individuals likely suffer negative welfare implications from being caught (with potential effects on their health and fitness), and researchers may waste valuable time and resources processing bycatch for release when they could be focusing on capturing and collecting data from target species. To remedy this problem, we introduce here a relatively simple trap design that installs on a bird feeder and traps birds using a wireless, remotely closing door to allow selective capture of birds visiting a feeding station, a common capturing context in ornithological studies. We describe how to construct the selective, remotely closing trap and report our experience using the traps to selectively capture house sparrows (Passer domesticus) in comparison to mist nets. This easy-to-use trap will benefit researchers looking to effectively and efficiently capture target species while also decreasing bycatch and will be especially convenient at established bird feeding stations.
This is the first in a series of papers that we expect to result from the house sparrow project. Future papers resulting from the project will focus directly on indicator validation and house sparrow welfare.
Visit our blog to read about our progress on the house sparrow project:
Research sense: Incorporating animals’ sensory capacities in animal care and study design
Mal Graham, Bob Fischer
Graham, M., & Fischer, B. (2025). Research sense: Incorporating animals’ sensory capacities in animal care and study design. Laboratory Animals. https://doi.org/10.1177/00236772251385784
Co-authored by Wild Animal Initiative Strategy Director Mal Graham, this paper was published online in Laboratory Animals in December 2025.
Abstract
No systematic procedures exist to ensure that differences in animal sensory capacities are accounted for in experimental design and ethical review processes. This oversight can compromise both scientific validity and animal welfare. This review presents three practical methodologies to address this gap: incorporation of specialist expertise through consultation frameworks, voluntary certification schemes modeled on Open Science practices, and mandatory sensory capacity review integrated into existing ethics committee processes. We provide a concrete tool — a sensory modality survey — that can be implemented by institutional review committees to evaluate sensory considerations in research proposals systematically. These approaches align with the 3Rs principles by enhancing experimental refinement and potentially reducing animal use through improved study design.
Rehabilitating wild animal welfare: A focus on veterinary rescue and rehabilitation interventions
Beaulieu, M. (2025). Rehabilitating wild animal welfare: A focus on veterinary rescue and rehabilitation interventions. Research in Veterinary Science, https://doi.org/10.1016/j.rvsc.2025.105582
Authored by Wild Animal Initiative’s Research Manager, Michaël Beaulieu, this paper was published in the April 2025 issue of Research in Veterinary Science.
Abstract
Conservation considerations are often put forward to justify wildlife veterinary interventions.
Paradoxically, the conservation impact of such interventions is often uncertain.
These interventions, however, have a clear impact on the welfare of wild animals.
This confusion is likely related to the connection between animal conservation and welfare.
Veterinarians need to explicitly recognize the welfare value of their interventions on wildlife.
Plugging biologging into animal welfare: An opportunity for advancing wild animal welfare science
Michaël Beaulieu, Michaela Masilkova
Beaulieu, M., & Masilkova, M. (2024). Plugging biologging into animal welfare: An opportunity for advancing wild animal welfare science. Methods in Ecology and Evolution, https://doi.org/10.1111/2041-210X.14441
Authored by Wild Animal Initiative Research Manager Michaël Beaulieu and Michaela Masilkova, Postdoctoral Researcher at Czech University of Life Sciences Prague, this paper was published in December 2024 in Methods in Ecology and Evolution.
Abstract
Animal welfare science is currently expanding beyond its traditional boundaries, from captive animals to those living in the wild. This current development is conceptually and methodologically challenging, but it could benefit from adjacent and more established research fields. Among these fields, biologging appears to be a strong candidate, as most intrinsic, location and environmental variables collected through biologging approaches could be used to assess animal welfare in the wild.
To provide an objective view of the suitability of biologging to assess wild animal welfare, biologging was evaluated against the criteria that are currently recommended to assess animal welfare. This evaluation shows that biologging approaches could enhance animal welfare assessments in terms of completeness, informativeness and feasibility in the wild. However, their full implementation may be complicated by limitations in terms of validity, representativeness and disturbance, and by the different welfare perspectives taken by wildlife biologists using biologging approaches and animal welfare biologists.
To exploit the full potential that biologging approaches could offer to assess wild animal welfare, their current limitations need to be overcome. Towards this end, recommendations are explicitly provided to enhance the validity and the representativeness of biologging measurements as welfare indicators, while reducing disturbance. To increase the visibility and the impact of biologging studies examining wild animal welfare, we also encourage wildlife biologists using biologging approaches to adopt the same language and perspectives as those used by animal welfare biologists.
If current limitations are overcome, biologging is likely to be instrumental for the future study of animal welfare in the wild. Reciprocally, integrating animal welfare in biologging studies is expected to have a great impact on the whole biologging field by extending its current scope to a new and promising research area.
Improving wild animal welfare through contraception
Simon Eckerström Liedholm, Luke Hecht, Vittoria Elliott
Eckerström Liedholm, S., Hecht, L., & Elliott, V. (2024). Improving wild animal welfare through contraception. BioScience, https://doi.org/10.1093/biosci/biae071
Authored by Wild Animal Initiative’s Researcher Simon Eckerström Liedholm, Science Director Luke Hecht, and Vittoria Elliott of the Smithsonian Institution, this paper was published September 11, 2024, in BioScience.
Abstract
To date, research on the welfare impacts of wildlife contraceptives has mostly been focused on the potential harms of contraceptives. However, there are compelling theoretical reasons to expect direct and indirect welfare benefits of wildlife contraceptives. These positive welfare effects would be experienced by more than just the treated individuals, because per capita resource availability will increase with decreasing numbers of individuals sharing a resource. In the present article, we discuss the potential for wildlife contraceptives to alleviate resource competition and their associated negative welfare effects at different scales. These effects are expected to vary across contexts and would presumably be stronger when wildlife contraceptives are used with the explicit purpose of improving wild animal welfare. The potential for considerable welfare gains for wildlife through the targeted use of contraceptives highlights the importance of both species-specific studies on the welfare benefits of wildlife contraceptives and further research on the links between population dynamics and wild animal welfare.
Oxidative status: A general but overlooked indicator of welfare across animal species?
Beaulieu, M. (2024). Oxidative status: A general but overlooked indicator of welfare across animal species? BioEssays, https://doi.org/10.1002/bies.202300205
Authored by Wild Animal Initiative Research Manager Michaël Beaulieu, this paper was published in August 2024 in BioEssays.
Abstract
Because of their ubiquity, plasticity, and direct effects on the nervous system, markers of oxidative status may be of great value to assess animal welfare across species and conditions in the wild. However, welfare biologists have not yet seized this opportunity, possibly because the validity of these markers as welfare indicators remains questionable. A validation process was, therefore, performed here using a meta-analytical approach considering three conditions assumed to impair the welfare of animals. With very few exceptions, two of the four considered markers consistently varied across these negatively-valenced conditions. By highlighting the current underrepresentation of markers of oxidative status in animal welfare studies, and by concretely illustrating that some of these markers can consistently reflect negative affective states, this article aims to encourage biologists to include these physiological markers in their toolbox to better measure, monitor, and perhaps also improve the welfare of animals in their natural habitat.
Validating physiological markers as welfare indicators: the case of oxidative stress
This research note is an extension of Michaël Beaulieu's paper, “Oxidative status: A general but overlooked indicator of welfare across animal species?,” which was published on June 4, 2024, in BioEssays’ “Problems & Paradigms” rubric.
Written by: Michaël Beaulieu
Published: August 30, 2024
DOI: https://doi.org/10.71441/42n6-4ata
Suggested citation: Beaulieu, M. (August 2024), Validating physiological markers as welfare indicators: the case of oxidative stress, Wild Animal Initiative, retrieved [date], https://doi.org/10.71441/42n6-4ata
This research note is an extension of Research Manager Michaël Beaulieu's paper, “Oxidative status: A general but overlooked indicator of welfare across animal species?,” which was published on June 4, 2024, in BioEssays’ “Problems & Paradigms” rubric.
Why physiological markers require validation as welfare indicators
Our previous article, “Capturing wild animal welfare: a physiological perspective,” described how physiological markers can most effectively and appropriately be used to assess the welfare of animals in their natural habitat. It offered the growing community of researchers interested in wild animal welfare science insights and guidance about the use of physiological markers according to theoretical principles. A key point of the article was that one of the main limitations of using physiological markers as welfare indicators is that the values of typical measurements of physiological markers taken from peripheral tissues like plasma may not be representative of the values found in the central nervous system, where affective states originate. Indeed, relying on peripheral measurements to assess animal welfare is problematic, as measurements taken from peripheral tissues may be affected by factors other than central processes and therefore do not necessarily (or only partly) reflect the affective states animals are experiencing. Despite this important limitation, researchers often implicitly assume that peripheral physiological markers reflect the welfare of animals. However, before any physiological or behavioral marker can be reliably used in animal welfare studies, an initial validation procedure is required to confirm its suitability as a welfare indicator. One such validation procedure was recently proposed, but has so far remained largely theoretical (Browning 2023).
Putting the validation process into practice
When applied to physiological or behavioral markers, the first three steps of the validation process can be formulated as follows:
Consider a range of conditions and postulate their effects on the valence of the affective states that would be experienced by animals when exposed to these conditions (i.e. whether it would elicit a positive or negative experience);
Measure the physiological or behavioral changes resulting from the exposure of animals to those conditions;
Examine the consistency of these physiological or behavioral changes across a variety of conditions assumed to similarly impact affective states (i.e. consistently positive or negative valence with high or low arousal), such that consistent changes are independent of the specific conditions affecting welfare, and instead reflect the expected change in valence and arousal.
Despite its simple logic, the application of this validation procedure may seem daunting. Indeed, it may be difficult to apply it in practice, as it requires measuring the effects of a variety of different conditions in a statistically relevant number of individuals distributed across several replicated populations (Beaulieu 2024). This important challenge can be overcome, however, by taking advantage of previous studies examining the effects of similarly valenced conditions on specific physiological or behavioral markers. This is the approach we used in a recently published study to evaluate the validity of markers of oxidative status as potential welfare indicators. In addition to assessing the representation of markers of oxidative status in the animal welfare literature, this study includes a meta-analysis based on the results of previous studies examining the effects of three conditions on the oxidative status of animals: social isolation, noise exposure, and predation exposure. These three conditions were expected to negatively affect the welfare of animals.
What is oxidative stress?
The presence of oxygen in the Earth’s atmosphere enables animals to produce the energy they need for their daily activities. However, the use of oxygen to produce energy can also result in the excessive production of molecules called Reactive Oxygen Species (ROS) that are capable of damaging important biomolecules such as proteins, lipids, and DNA. To counteract the effects of ROS, animals have developed complex defense machinery composed of a variety of antioxidant molecules, which allows them to minimize oxidative damage. This defense machinery has limits, however, and antioxidant defenses may sometimes be overwhelmed by ROS production. This can lead to oxidative stress: an unbalanced oxidative status between ROS and antioxidant defenses in favor of ROS that leads to high levels of oxidative damage (Costantini & Verhulst 2009). Importantly, not all tissues and organs are equal in terms of oxidative stress. For instance, compared to most organs, the brain is more likely to experience oxidative stress because of the high level of energy it requires, the high levels of ROS it produces, its low endogenous levels of antioxidant compounds, and its overall biochemical composition (Salim 2017). In humans and in laboratory rodents, negatively valenced affective states like irritability, anxiety, and depression have repeatedly been associated with high levels of oxidative damage in the brain (Hovatta et al. 2010). The fact that wild animals likely experience comparable affective states suggests that their welfare could also be reflected by and assessed through markers of oxidative status.
Is oxidative stress being measured in animal welfare studies?
Despite the potential for markers of oxidative status to be used in welfare studies, the results of a review conducted in three animal welfare journals publishing research articles over the last decade (the “Animal Welfare” section of Animals, the Journal of Applied Animal Welfare Science, and Animal Welfare) show that so far, only 5% of studies have considered these markers for directly assessing the welfare of animals. Across the 295 studies reviewed, markers of oxidative status were unevenly represented, and the selection of given markers of oxidative status in these studies appears largely subjective (or at least not explicitly justified). Moreover, these markers were mainly measured in captive mammals and birds experiencing a low variety of artificial conditions (unlike studies in the adjacent fields of ecophysiology and conservation physiology, which cover a broader variety of markers, conditions, and taxa). Only one study used markers of oxidative status to explicitly assess the welfare of wild animals (wild boars in Esposito et al. 2021). The relative rarity of markers of oxidative status in the current animal welfare literature may, at least in part, be the result of not having undergone a validation process to confirm their reliability as welfare indicators. This validation is all the more important for wild animals, as markers of oxidative status are typically measured in their peripheral tissues and not directly in their nervous system (Beaulieu 2024).
Applying a validation procedure to markers of oxidative status
Four markers of oxidative status in response to noise exposure, social isolation, and predation exposure were found to be represented in the published literature at a level sufficient for use in the meta-analysis. To avoid the effects of potential confounding factors, all of the studies considered were experimental and conducted under controlled conditions with domesticated animals (laboratory rodents exposed to noise or social isolation) or wild animals studied in captivity (insect larvae, crustaceans, and tadpoles exposed to predatory cues). The results of this meta-analysis show that, with very few exceptions, two of the four considered markers of oxidative status consistently vary irrespective of the nature of the conditions negatively affecting the welfare of animals. These are the levels of malondialdehyde (a marker of oxidative damage on lipids) increase and the levels of glutathione (an endogenous antioxidant marker) decrease. The two antioxidant enzymes did not respond in a consistent manner, even within each considered condition. When both peripheral and central measurements were available (as in the case of noise exposure), peripheral measurements mostly reflected central measurements. Altogether, these results indicate that some peripheral markers of oxidative status could be considered as valid indicators of animal welfare, contrasting with their underrepresentation in the current animal welfare literature.
Conclusions and perspectives
This study provides information about the potential use of markers of oxidative status as welfare indicators. It also illustrates how the process of examining the validity of physiological markers as welfare indicators can be implemented, even without conducting new studies. Importantly, the validation process used here for markers of oxidative status is not restricted to physiological markers, but could also be extended to test the validity of behavioral markers as welfare indicators (Browning 2023). Moreover, the assessment of physiological and behavioral markers as welfare indicators could be conducted simultaneously to examine their interrelationships and how they each relate to certain welfare dimensions like valence, arousal, and persistence. For instance, the assessment of markers of oxidative status as welfare indicators could be conducted at the same time as the assessment of vocalizations, which are known to be affected by oxidative stress and potentially reflect animals’ welfare (Briefer 2012; Casagrande et al. 2016).
Making use of historical datasets by using meta-analytical approaches as we did here obviates the need to disturb additional animals to validate new welfare indicators. This convenient and ethical approach is consistent with the 3Rs (Reduce, Replace, Refine) approach currently recommended in animal experimentation (NC3Rs). A drawback of this meta-analytical approach, however, is that it limits the scope of the validation process to the species, conditions, and physiological markers that are already available in the published literature. Other conditions and physiological markers that have not yet been studied may also be worth examining, especially when working with species underrepresented in the scientific literature, such as many invertebrates. For instance, in the case of oxidative status, some markers could not be considered in the validation process conducted here because of their low representation in the current literature. Moreover, because the available literature focuses more strongly on negatively valenced conditions than on positively valenced ones (Nelson et al. 2023), it was not possible to assess their validity as indicators of positive welfare based on a meta-analytical approach. This is an important limitation, both in terms of markers and conditions affecting welfare, as there is evidence that some markers of oxidative status might also reflect positive affective states (Cafazzo et al. 2014). Finally, indication of publication bias — the propensity to publish results based on their direction, as was sometimes found in this meta-analysis — may cast some doubts on the results of meta-analyses. Overall, these limitations suggest that it is necessary to complement validation procedures based on meta-analytical approaches with field or lab work, despite the related workload and potential costs. These additional empirical studies, some of which may use harmful methods, should only be considered acceptable if they advance our knowledge sufficiently by moderately impacting the few individuals under scrutiny while strongly benefitting the many others living in the wild. Funding agencies therefore need to be convinced of the necessity to validate potential welfare indicators and to allocate substantial amounts of money to financially support such challenging validation projects. We hope that the recent publication of several articles highlighting this urgent need (Beaulieu 2024; Browning 2023), as well as this new study on the potential use of markers of oxidative status in animal welfare studies, will help make this happen and allow us to better assess the welfare of wild animals in the future.
Capturing wild animal welfare: A physiological perspective
Beaulieu, M. (2024). Capturing wild animal welfare: a physiological perspective. Biological Reviews, https://doi.org/10.1111/brv.13009
Authored by Wild Animal Initiative Research Manager Michaël Beaulieu, this paper was published in February 2024 in Biological Reviews.
Abstract
Affective states, such as emotions, are presumably widespread across the animal kingdom because of the adaptive advantages they are supposed to confer. However, the study of the affective states of animals has thus far been largely restricted to enhancing the welfare of animals managed by humans in non-natural contexts. Given the diversity of wild animals and the variable conditions they can experience, extending studies on animal affective states to the natural conditions that most animals experience will allow us to broaden and deepen our general understanding of animal welfare. Yet, this same diversity makes examining animal welfare in the wild highly challenging. There is therefore a need for unifying theoretical frameworks and methodological approaches that can guide researchers keen to engage in this promising research area. The aim of this article is to help advance this important research area by highlighting the central relationship between physiology and animal welfare and rectify its apparent oversight, as revealed by the current scientific literature on wild animals. Moreover, this article emphasises the advantages of including physiological markers to assess animal welfare in the wild (e.g. objectivity, comparability, condition range, temporality), as well as their concomitant limitations (e.g. only access to peripheral physiological markers with complex relationships with affective states). Best-practice recommendations (e.g. replication and multifactorial approaches) are also provided to allow physiological markers to be used most effectively and appropriately when assessing the welfare of animals in their natural habitat. This review seeks to provide the foundation for a new and distinct research area with a vast theoretical and applied potential: wild animal welfare physiology.
Quantifying the neglectedness of wild animal welfare
Michaël Beaulieu's deep dive examines the quality and quantity of mentions of wild animal welfare in the scientific literature and finds it to be a neglected research area.
Written by: Michaël Beaulieu
Published: November 22, 2023
DOI: https://doi.org/10.71441/7vws-rn93
Suggested citation: Beaulieu, M. (November 2023), Quantifying the neglectedness of wild animal welfare, Wild Animal Initiative, retrieved [date], https://doi.org/10.71441/7vws-rn93
Questioning the neglectedness of wild animal welfare: passionate claims, illusionary facts, or simple truth?
Wild animal welfare is often suggested to be a neglected research area, worthy of more attention from scientists and policy makers (e.g., Tomasik 2015; Forristal 2022). Considering the general propensity of animal welfare to unleash passions even among scientists (Coghlan & Cardilini 2022), it is important to assess the veracity of these claims. So to objectively assert the need to conduct more research to better understand wild animal welfare and implement sound welfare interventions in the wild, clear evidence (or “stubborn facts” insensitive to people’s inclinations and passions; Adams 1770) supporting the neglectedness of wild animal welfare is required.
Cursory research of the expression “wild animal welfare” in Google Scholar (Nov. 2023) provides 844 outputs, only 7% of which emphasize the expression in their title or abstract. However, such figures may be misleading if wild animal welfare is already considered under alternative synonyms or already incorporated into adjacent disciplines. For instance, the fate of wild animals in response to variable environmental conditions has traditionally been under the scrutiny of conservation practitioners (Brown 2007; Nijhuis 2020). Because conservation studies typically focus on conditions that affect animals in their natural habitat (for instance, environmental conditions changing because of anthropogenic activities and that may be perceived as unpleasant by wild animals), they may theoretically also encompass aspects related to wild animal welfare (Sekar & Shiller 2020). Therefore it is possible wild animal welfare is not so highly neglected, but rather often concealed within existing animal conservation studies. Importantly, because the multidisciplinary field of wild animal welfare includes a variety of approaches, indicators, and taxa, some research areas within this field may be more neglected than others.
Assessing the neglectedness of wild animal welfare
To assess the neglectedness of wild animal welfare, I have conducted a targeted review of the literature in peer-reviewed journals publishing research in animal welfare (three journals: Animal Welfare, Journal of Applied Animal Welfare Science, animal welfare section of Animals) and animal conservation (five journals: Animal Conservation, Biological Conservation, Conservation Biology, Conservation Physiology, Oryx). I selected these journals because of their high visibility and their representativeness in their respective field. I considered a date range of 2013-2022 for the review process, in order to reflect contemporary research on animals. Accordingly, I only included research articles (as opposed to reviews, commentaries) focusing on animals (as opposed to, e.g., plants, policy) in the review of both fields. I identified and classified the study subjects of each welfare science article as “wild” if they were studied in their natural habitat or collected in the wild and then studied in captivity. In conservation articles, I searched for welfare-related terms (e.g., welfare/wellbeing, sentience, emotion, conscious, subjective experience, affective state, compassionate) in both the title and abstract.
Confirming the neglectedness of wild animal welfare
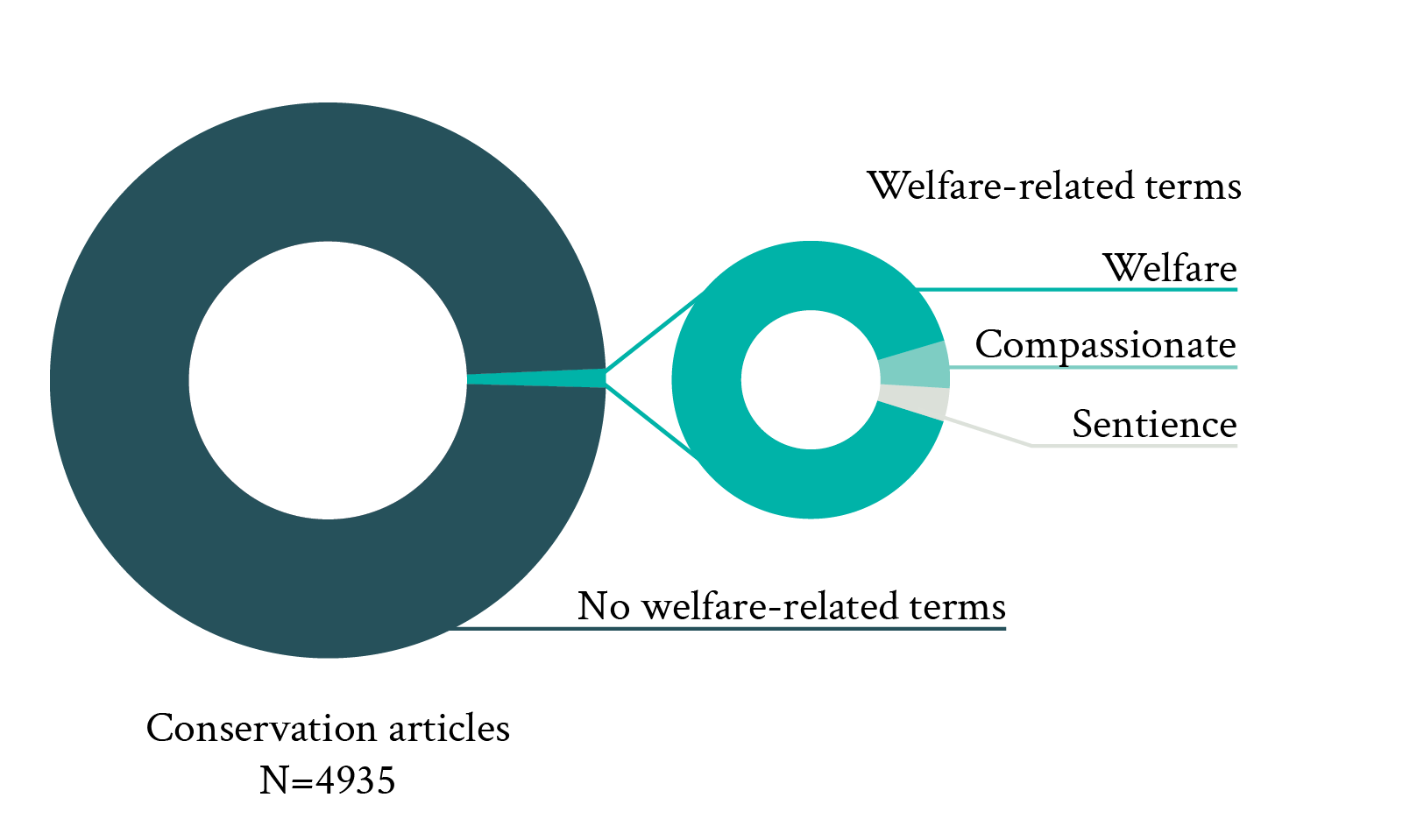
Both the presence of wild animals among all reviewed welfare studies (6%) and the occurrence of welfare-related terms in conservation studies (1%) were very low (Fig. 1). Among welfare-related terms, “welfare/wellbeing” was by far the most commonly used term (90%) in conservation journals. In addition to “welfare/wellbeing,” other welfare-related terms such as “compassionate” (6%) and “sentience” (4%) were also found. However, other welfare-relevant terms such as “conscious,” “subjective experience,” or “affective state” were completely absent. Altogether, these proportions strongly suggest that wild animal welfare is neglected by both the welfare and conservation literature.
Figure 1. Proportion of articles published in three principal animal welfare journals between 2013 and 2022 and including wild animals (left pie, top on mobile), and proportion of articles published in five principle conservation journals over the same period and including welfare-related terms in either their title or abstract (welfare, compassionate and sentience were the only terms found; right panel, bottom on mobile).
Dissecting the neglectedness of wild animal welfare: the example of physiological approaches
To understand how an animal experiences their life in the wild, it is necessary to use approaches that can evaluate and provide evidence of their welfare. Behavioral and physiological markers potentially represent downstream indicators of welfare, and can thus provide insight into the welfare of wild animals (Browning 2022). I focused here on physiological markers specifically, as they may be highly informative (on their own or to complement behavioral approaches) for assessing the welfare of wild animals (see deep dive: Welfare and physiology: a complicated relationship). To quantify the degree to which physiological approaches are also being neglected in wild animal welfare science, I have further examined the welfare science studies previously identified as being conducted on wild animals to determine whether they made use of physiological markers and in which species.
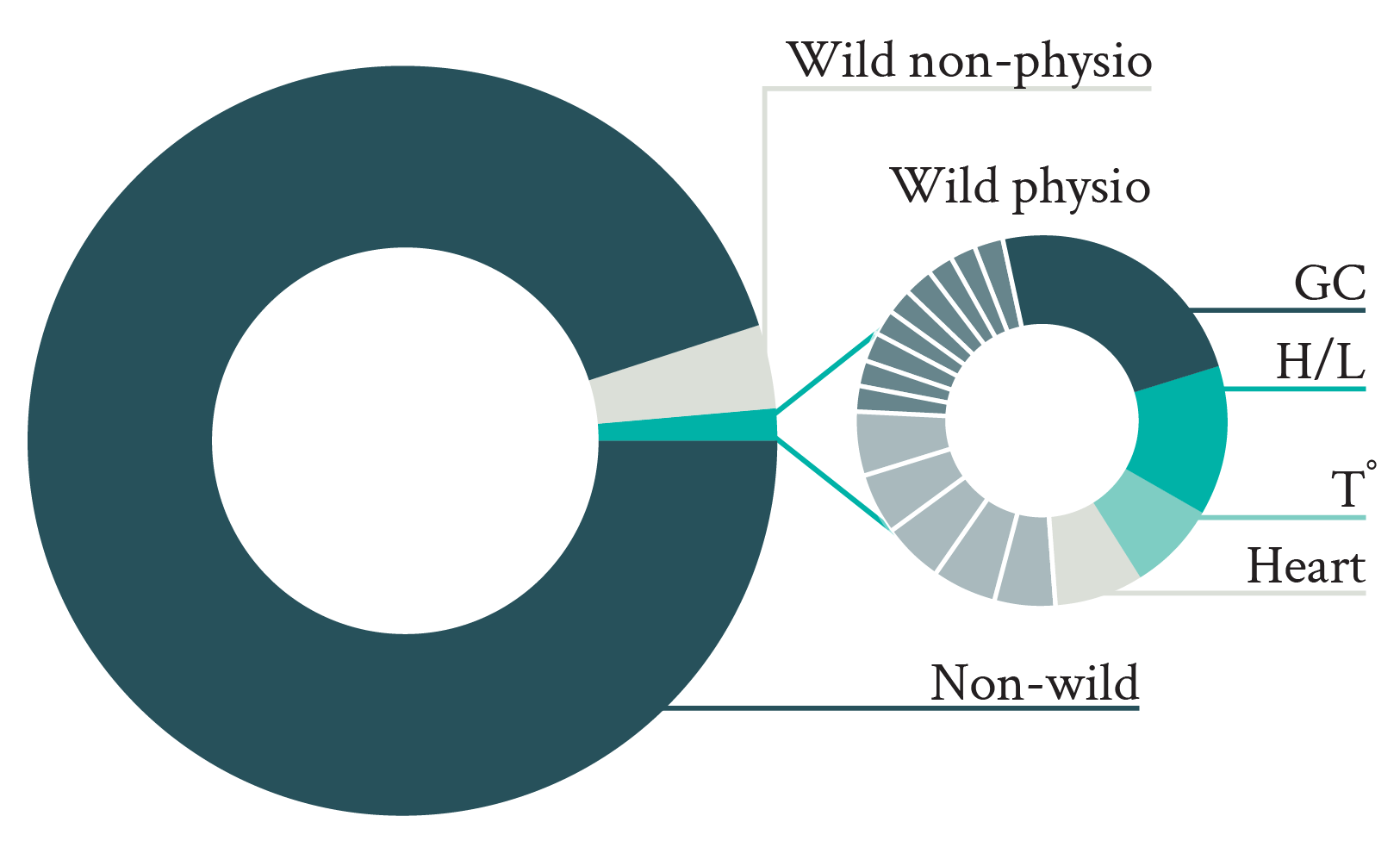
Only 22% of welfare science studies conducted on wild animals included the use of physiological markers (Fig. 2). These results suggest that wildlife biologists either are reluctant to make use of physiological markers in their research relative to other welfare indicators (e.g., living conditions, behavior), or they lack awareness of how to incorporate them. Yet the very existence of the field of conservation physiology, and the numerous studies published, demonstrate that it is possible to measure a diversity of physiological markers in the wild to examine how wild animals are affected by given environmental conditions (Cooke et al. 2013). Nevertheless, despite this potential, thus far such studies have not focused on assessing welfare, as indicated by only 3% of articles published in conservation journals and including physiological parameters mentioning welfare-related terms in their title or abstract. Moreover, the few studies that considered physiological parameters to assess welfare in wild animals focused on a limited number of physiological markers (compared to the vast variety of physiological parameters that could theoretically be measured to assess welfare; e.g., Jerez-Cepa & Ruiz-Jarabo 2021; Whitehead & Dunphy 2022) measured in few taxa, the typical study measuring glucocorticoids in a mammal species (Fig. 2).
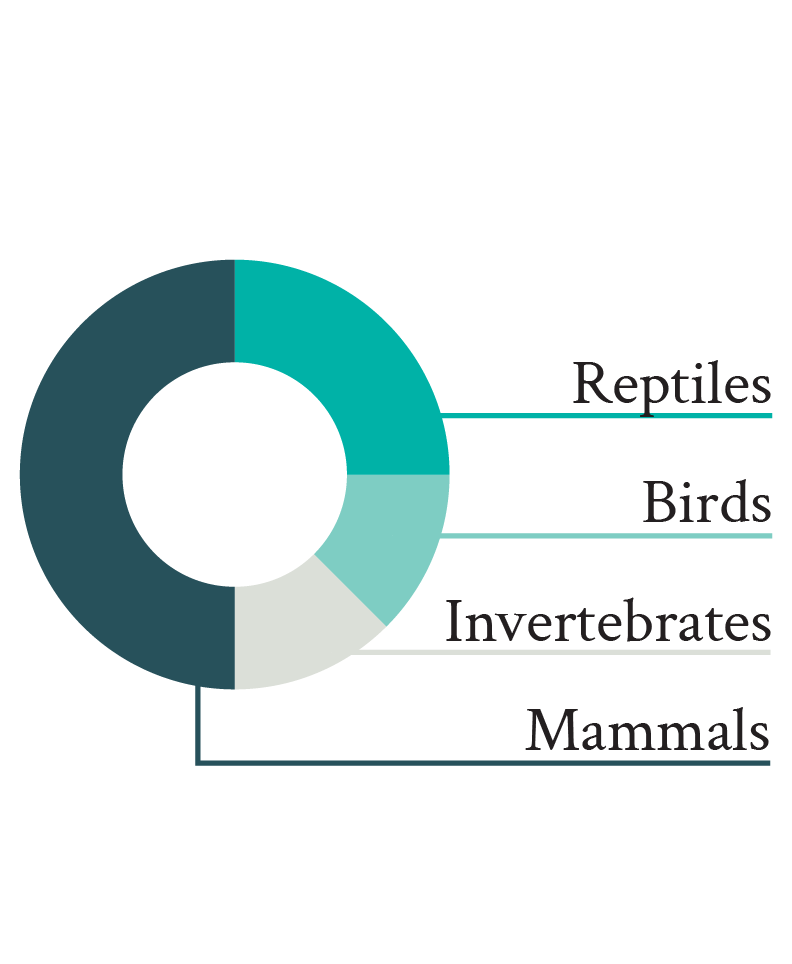
Figure 2. Proportion of articles published in three principal animal welfare journals between 2013 and 2022 and including wild animals (left pie, top on mobile) and measuring physiological markers (striped area). The detail of the measured physiological markers (GC: glucocorticoids; H/L: heterophil/lymphocyte ratio, Tº: temperature) and in which taxa (Invert.: invertebrates) they were measured is illustrated in the right pies (bottom on mobile). For clarity, only the first half of the physiological markers measured in articles on wild animal welfare physiology is annotated.
The overrepresentation of glucocorticoids (and their effects on the H/L ratio; Davis et al. 2008) and mammals among studies on the physiology of wild animals is not necessarily surprising, but their measurement is questionable when examining their welfare. Indeed, even though measuring glucocorticoids reflects a general trend observed in animal stress physiology (MacDougall-Shackleton et al. 2019), the relationship between glucocorticoids and welfare remains unclear (Ralph 2016). Therefore, for glucocorticoid measurements to offer useful insights about welfare, they need to be complemented by additional markers (e.g., behavioral observations). Moreover, even though the imbalance in favor of mammals probably reflects the widespread greater interest in these animals, mammals are relatively rare in nature (ca. 0.1% of all animal species and ca. 0.35% of the whole animal biomass on Earth; Mora et al. 2011; Burgin et al. 2018; Bar-On et al. 2018), and the way they experience their lives is probably not comparable to that of other taxa with very different neural organizations (e.g., more common invertebrates; Paul et al. 2020).
Conclusions
Review of recent literature in key peer-reviewed journals in animal welfare showed that the neglectedness of wild animal welfare is indeed a “stubborn fact” and not just a questionable claim. Importantly, the targeted review of journals in animal conservation also provided evidence that this underrepresentation was not simply an artifact of the inclusion of welfare aspects within animal conservation studies (that were found to refer to animal welfare only anecdotally and superficially). Moreover, within the limited number of studies that did focus on wild animal welfare, the limited use of physiological markers further highlights the neglectedness of valuable methodological approaches and taxa. If allowed to persist, such biases will likely limit our understanding of wild animal welfare, as the strength of this field precisely lies in its diversity of approaches and the range of taxa to which they can be applied. Continuing not to take advantage of such potential would be short-sighted.
The limited availability of welfare science studies focusing on wild animals suggests that there may be resistance or barriers to extending the scope of welfare science beyond the traditional context of farmed, companion, and captive-housed animals. A contributing factor may be that assessing welfare is easier in well-known model species maintained under controlled conditions in captivity (e.g., pigs), compared with their wild counterparts living under uncontrollable conditions (e.g., wild boars). Another contributing factor might also be related to the uncertainty about the capacity of many wild animals to have subjective experiences (e.g., invertebrates, but see Anderson & Adolphs 2014; de Waal & Andrews 2022). Despite these inherent challenges, there is increasing evidence that extending the field of animal welfare to wild conditions is feasible (Harvey et al. 2020), particularly through collaborations among scientists from a range of disciplines, and could have far-reaching impacts for our general understanding of how most animals on Earth experience their lives. In addition to advancing general understanding and increasing available knowledge, wild animal welfare studies may also lead to concrete applications — for instance, by informing stakeholders, refining practices, or modifying legislation impacting wildlife. But the full potential of the field of wild animal welfare will be reached only if scientists branch out beyond traditional norms and consider the full range of approaches and taxa that this field has to offer.
Welfare and physiology: a complicated relationship
This research note is an extension of Michaël Beaulieu's paper, Capturing wild animal welfare: a physiological perspective, which was published August 27, 2023, by Biological Reviews online.
Written by: Michaël Beaulieu
Published: September 18, 2023
DOI: https://doi.org/10.71441/ssh5-4fft
Suggested citation: Beaulieu, M. (September 2023), Welfare and physiology: a complicated relationship, Wild Animal Initiative, retrieved [date], https://doi.org/10.71441/ssh5-4fft
This research note is an extension of Research Manager Michaël Beaulieu's paper, Capturing wild animal welfare: a physiological perspective, which was published August 27, 2023, by Biological Reviews online.
Short-term emotions and long-term moods correspond to affective states that, depending on the time scale being considered, determine how an animal experiences their life (Crump et al. 2018). In vertebrates, the generation of affective states depends on the relative levels of neurotransmitters (e.g. dopamine, serotonin, etc.) in specific regions of the brain (Lövheim 2012). Despite very different neural organization, most of these neurotransmitters are also found in invertebrates (with or without a central nervous system), in which they also appear to be involved in the expression of affective states (Bateson et al. 2011; Fossat et al. 2014; Perry et al. 2016).
The production of neurotransmitters responsible for related affective states is triggered by external stimuli and requires information processing (e.g., exposure to predators triggering fear) and can, in turn, affect the whole peripheral physiology of the individual. In vertebrates, the transmission of affective states from central neural structures to the periphery of the individual occurs through the modulation of the autonomic nervous system and the hypothalamo-pituitary-adrenal axis (Stiedl et al. 2010; Keifer et al. 2015; Zhang 2021). Rapid transmission of affective states to the periphery is expected to be highly adaptive, as it likely helps animals to adopt the most relevant physiological and/or behavioral response (fight/flight vs. rest/digest) to the conditions they encounter. For instance, if an individual experiences fear because of the presence of a predator, adopting a fight or flight strategy is likely to promote survival, assuming they have the capacity for combat or escape (Doherty & Ruehle 2020). Such a response is associated with a sudden increase in energetic requirements and is expected to be accompanied: (1) At the physiological level, by the breakdown of stored macromolecules (e.g. glycogen, triglycerides, lipids) into usable smaller molecules (e.g. glucose, fatty acids, glycerol), their release into the bloodstream, and the inhibition of glucose uptake by peripheral organs; (2) At the functional level, by faster heart and respiratory rates, higher core temperature, and the redistribution of blood flow to priority organs (e.g. heart, brain, etc.).
Peripheral physiological and functional changes may themselves also affect the expression of affective states. For instance, laboratory mice for whom heart rate has been experimentally increased can experience anxiety-like states in risky contexts (Hsueh et al. 2023). Such peripheral effects on the expression of affective states may be mediated by a variety of physiological factors (e.g. hormones, inflammatory factors crossing the blood-brain barrier) and likely contribute to interoceptive processes (i.e., experiencing the physiological condition of the body, such as temperature or pulse; Chen et al. 2021). Therefore the connection between the affective states experienced by animals and their peripheral physiology is bidirectional. In other words, the affective states experienced by animals (e.g. anxiety) can affect their peripheral physiology (e.g., heart function) and, conversely, their peripheral physiology can also affect their subjective experience. Importantly, affective states and peripheral physiological markers (i.e. ‘measurable indicators of the body’s physiological status’; Jesuthasan et al. 2022) may theoretically covary even in the absence of direct interactions between them if they are both affected by the same external factors. Peripheral physiological parameters may therefore reflect affective states both in a causal and in a correlative way.
The challenge of using physiological markers as welfare indicators
In the same way that wildlife biologists measuring physiological markers in other disciplines (e.g. ecophysiology, conservation physiology) have access only to peripheral physiological markers, biologists wishing to use physiological markers to assess the welfare of animals in the wild are also typically limited to peripheral measurements. This results from both limited availability of suitable techniques and limitations of the techniques currently available for directly measuring physiological processes that are occurring in the brain of free-ranging animals, the use of which would also raise important ethical concerns (Gaidica & Dantzer 2022). Peripheral physiological markers can also be measured in other tissues besides those that can be sampled directly from animals that have been captured (e.g. blood, muscles), including peripheral structures (e.g. hair, feathers) and excretory material (e.g. feces) left by animals in their environment.
Because of the bidirectional relationship between affective states and physiology described above, any peripheral physiological marker affected by and/or affecting the expression of affective states could theoretically be used as an indicator to assess the welfare of wild animals. However, peripheral physiological parameters are not only involved in the expression of affective states but also in other processes, such as the regulation of homeostasis (i.e., “a self-regulating process by which biological systems maintain stability while adjusting to changing external conditions”; Bilman 2020). Importantly, homeostatic changes may be but are not necessarily related to changes in affective states. For instance, being dehydrated leads both to physiological changes aiming at correcting this imbalance (e.g., production of antidiuretic hormone) and to thirst (i.e., an affective state motivating water intake). In contrast, when the conditions with which the organism has to cope require an immediate response without interpretation by animals, peripheral physiological processes aiming at correcting homeostasis may be activated independently of any emotional processes when (e.g., hypoglycaemia; Herman & Culliman 1997). Hence, most peripheral physiological parameters likely reflect to some extent both the emotional and the homeostatic states of the organism, which are intermingled but not necessarily interconnected. Finally, because peripheral physiological functions constantly interact with each other, the relationship between a given peripheral physiological marker and a given affective state is likely to be blurred by the interactions that this specific physiological marker has with other physiological functions (themselves affected or not by emotional and/or homeostatic conditions). This complexity points to the need to develop methodological approaches to validate the use of physiological parameters as robust indicators of animal welfare.
Validating physiological markers as welfare indicators in the wild
Most wildlife biologists already measuring physiological markers appear to select markers based on their own knowledge, background, and experience. For example, in the field of ecophysiology or conservation physiology, plasma glucocorticoids are traditionally (rightly or wrongly) measured to assess how vertebrates cope with challenging conditions because of their implication in the stress response (Beaulieu & Costantini 2014, MacDougall-Shackleton et al. 2019). Assuming that glucocorticoid levels also reflect certain affective states, the choice to measure glucocorticoids in welfare studies would imply that (1) the considered affective states and glucocorticoids vary on the same time scale (Gormally & Romero 2020), and (2) the relationship between given affective states and glucocorticoids is predominant over any homeostatic influences affecting glucocorticoid levels. Typically these assumptions (and the choice of glucocorticoids) in welfare studies are not well justified, which has presumably contributed to question the relevance of using such markers to assess animal welfare despite their direct relationship with centrally-generated affective states (Ralph & Tilbrook 2016). The widespread use of glucocorticoids without clear evidence of the mechanisms and how to interpret results clearly demonstrates that peripheral physiological markers need to be validated before being convincingly used as welfare indicators.
Based on Browning 2023, the validation process for peripheral physiological markers as welfare indicators could be divided into three consecutive steps:
Formulating and articulating assumptions about the valence and arousal value of the affective states experienced by animals under certain conditions (e.g. a negative valence and an increased arousal assumed to result from predator exposure);
Measuring whether these conditions are associated with a change in the peripheral physiology of animals (e.g. higher glucocorticoid levels observed in animals exposed to a predator);
Ensuring that this peripheral physiological change is consistent across a variety of conditions assumed to affect welfare in a similar fashion (e.g. increased glucocorticoid levels observed irrespective of the nature of the predator and in socially-isolated or restrained animals).
If a given physiological marker consistently varies across conditions all assumed to be associated with the same valence and arousal, then it could be considered as a robust indicator of welfare (as its variation is independent of the condition causing the assumed welfare change).
Even though the proposed validation process may appear quite intuitive and simple, it may be difficult to put into practice, especially in the wild. Indeed, the last validation step implies the use of a replication approach based on the examination of different populations exposed to different conditions or the same populations sequentially exposed to different conditions (as recommended in ecological studies; Filazzola & Cahill 2021). This rigorous scientific approach may, however, be impracticable in the wild because of the associated increased costs, workload, and the potential disturbance generated by multiple animal manipulations. This limitation points to the need during the validation process to first consider the feasibility of including collection of biological samples, and adopting a replication approach.
To facilitate this validation process, it might also be possible to apply it first to non-wild animals who might be better amenable to replication approaches, and for whom the conditions they experience can be better controlled. In that case, it might be possible to work with individuals of the same species as the species of interest in the wild, or those of a closely-related species — typically the subjects of laboratory studies (e.g. rodents, fruit flies) or among the many species kept in zoos. Researchers, who choose to apply the validation process to non-wild animals first before applying the results in the wild, must nevertheless be mindful that the physiology and behavior of non-wild animals may not necessarily be comparable to that of wild animals (Crates et al. 2023). At the very least, non-wild individuals may still be studied for the analytical, physiological and biological validation of particular physiological measurements (Palme 2019). Moreover, non-wild individuals could also be studied to examine the relationship between specific physiological markers and behavioral welfare indicators that have been previously established based on sophisticated behavioral approaches to directly reflect emotional valence. Such sophisticated behavioral approaches would be difficult or even impossible to use in the wild (e.g. cognitive biases; Crump et al. 2018).
Conclusion
The relationship between welfare and physiology is highly complex because it can be causal/correlational, direct/indirect, and bidirectional, which makes the use of peripheral physiological markers to assess wild animal welfare challenging. Even without knowing the exact nature of the relationship between peripheral physiological markers and affective states, it is still possible to apply a validation process to examine whether physiological markers can be used as reliable welfare indicators. By determining consistent relationships between markers and affective states across conditions, it is indeed possible to establish them as robust welfare indicators that could then be combined with other validated markers to obtain a more thorough welfare profile.
Many welfare biologists could be discouraged from applying the proposed validation process because it is based on various initial assumptions and requires such a demanding replication approach. Nevertheless, this challenge may be overcome by collaborations between welfare biologists and physiologists, wildlife biologists, or zoo practitioners. Importantly, once validated, physiological markers have several advantages for assessing wild animal welfare that may convince welfare biologists to use them:
Physiological measurements are generally considered to be highly objective and straightforward.
Physiological responses can occur in the absence of any observable behavioral response.
Physiological responses can be measurable in some tissues at a later time, following an event that affects welfare, thereby possibly reflecting affective states across time.
Finite physiological responses with pleiotropic (or multiple) effects on behavior, although variable, are likely to be more consistent than behavioral responses between species, thereby facilitating comparative welfare studies.
As such, welfare biologists would benefit from adding validated physiological measures to their toolbox, as this may help them to draw a more holistic picture of the affective states experienced by animals in the wild.
Wildlife contraception and welfare
Simon Eckerström Liedholm discusses why contraception is a promising tool for improving wild animal welfare, and what further research is most urgently needed.
Written by: Simon Eckerström Liedholm
Published: April 8, 2022
DOI: https://doi.org/10.71441/1kqv-dbgz
Suggested citation: Eckerström Liedholm, S., (April 2022), Wildlife contraception and welfare, Wild Animal Initiative, retrieved [date], https://doi.org/10.71441/1kqv-dbgz
Contraception as a promising tool for welfare improvements
When resources are scarce, wild animals sometimes die of starvation (Gordon et al. 1988), dehydration, or exposure due to a lack of suitable shelters (Sibly & Hone 2002, Hill et al. 2019), all of which cause significant suffering (Gregory 2008). In many species, the competition for resources is especially strong among juvenile animals (Sol et al. 1998, Ward et al. 2006). Naively, making more resources available seems like it would solve the resource scarcity problem, but increasing the amount of available resources will often lead to higher fertility rates and juvenile survival rates, which eventually leads to larger populations instead (Prevedello et al. 2013, Ruffino et al. 2014), such that the competition for resources does not decrease in the long term.
One simple approach to find out how many animals are negatively affected by resource scarcity is to measure the fraction of deaths that are directly attributed to starvation. However, such an approach plausibly underestimates the pervasiveness of resource scarcity, due to interactions among different types of causes of death. For example, predation appears to be the most common cause of death for several taxa and life stages (Hill et al. 2019, Collins & Kays 2011), but whilst it is the proximate or final cause when it happens, it may not always be the ultimate cause — the underlying reason the lethal event occurred. For example, an animal that is starving may be prone to taking risks (Anholt & Werner 1995), or too weak to run away, which causes them to be more exposed to predators, making them more likely to be eaten. Therefore, even for some proximate causes of death that do not seem to be related to resource scarcity, there could be an underlying scarcity-related risk factor. In that case, a reduction of one cause of mortality (e.g. predation) might be partially or fully ‘compensated’ by the increase of another cause of mortality (e.g. starvation) such that life expectancy doesn’t change, known as compensatory mortality (Bergman et al. 2015). If everyone who is starving is eaten by a predator just before they would otherwise die of starvation, it would superficially appear like starvation wasn’t a problem at all, if we base our judgment solely on the proximate cause of death (predation in this case). Nevertheless, resource scarcity does appear to be a fairly common phenomenon (Prevedello et al. 2013), and irrespective of the ultimate cause of death, living a life of near starvation is a negative experience and of welfare concern. However, since resource limitations often are restraining population sizes (Prevedello et al. 2013), many populations will simply grow in response to increased resource availability, resulting in a larger population with similar levels of starvation.
Contraception provides one mechanism by which resource scarcity could be reduced, without inducing a corresponding increase in population size (Hecht 2021). Moreover, contraception may improve individual well-being through the alleviation of resource scarcity in other ways as well. For example, contraception could improve the welfare of the offspring by reducing sibling competition for resources (Mendl 1988, Andersen et al. 2011, Hudson et al. 2011). It could also reduce the burden of gestation and childrearing for some parents by allowing them to save energy and invest more in maintaining body condition (Kirkwood 1977, Kirkwood & Rose 1991, Lemaître et al. 2015). The cost of parental care, and thus benefits of reducing it, are dependent on the species and sex of the individual (Goldberg et al. 2020, Santos & Nakagawa 2012), which will cause additional variation in the net welfare effect of contraception.
There are several studies in which the effects of contraceptives on proxies for welfare have been investigated in wild animals (see Supplementary Table 2 in the review by Gray & Cameron 2010), and they seem to show both negative and positive welfare effects. The negative effects that were detected for some species/methods are related to maladaptive social behavior and inflammation at injection sites, among other things (Gray & Cameron 2010). The plausibly positive effects that have been found are related to survival and body weight, which are the types of benefits that we would predict given the model outlined in the previous paragraph. Several of the studies on survival detected increases (see for instance: Twigg et al. 2001, Turner & Kirkpatrick 2002, Williams et al. 2007), but one of them found no effect (Saunders et al. 2002), and two of them observed mixed results (Ransom et al. 2013 and Bromley & Gese 2001). Many studies have looked for changes in body weight, and several of them have found increases in treated females (see Table S2 in Gray & Cameron 2010). Such increases in body weight could serve as a proxy for welfare through improved nutritional status, but it could also be a reflection of changes in satiety and the foraging risk-reward trade off, where the ultimate effect on welfare is less clear. Many studies have had very small sample sizes, lacked control groups, and focused on a narrow set of welfare relevant traits, so it is hard to draw any strong conclusions. However, it seems very likely that the type, target and delivery mechanism of contraceptives have contributed to the observed differences in welfare-related outcomes among studies.
It is important to note though, that wildlife contraceptives haven’t been developed or applied with the goal of improving wild animal welfare by alleviating resource scarcity. As an analogy: even though a squirt gun is ineffective at putting out large fires — it was never intended for that use — water delivered in the right way is nonetheless very useful when there is a fire. Similarly, it is perhaps no surprise that the currently available evidence on the welfare effects is inconclusive, if no one has intentionally tried to improve wild animal welfare with it. It is the compelling theoretical potential that wildlife contraception offers for significant welfare improvement for large numbers of individuals that makes us excited to see further research in this area.
A very brief history of wildlife contraception research
As of 2011 (Kirkpatrick et al. 2011), wildlife contraception had been developed and successfully tested in 85 species of animals (mostly mammals), and work on different forms of wildlife contraception have continued since then (Asa & Moresco 2019). There are several different forms of contraceptives available for wild animals. Vaccine-based contraceptives (so-called immunocontraceptives) like the porcine zona pellucida (PZP) vaccine and the gonadotropin-releasing hormone (GnRH) vaccine were developed for feral horses and other wild mammals in the 1980s, and are still being used today (Asa & Moresco 2019). Steroid hormonal contraception methods have also been investigated, although high costs, toxicity, and problems with delivery (Kirkpatrick et al. 2011) have caused these methods to mostly be abandoned. Surgical sterilization has also been used to reduce fertility in wild populations of animals (Denicola & Denicola 2021), but because of the invasiveness of the procedure, it is likely more detrimental to animal welfare than other methods (Hampton 2017). It is also oftentimes more expensive per treated animal (Kirkpatrick et al. 2011). More recently, contraceptives have been developed for birds (Nicarbazin), such as pigeons and geese, and 4-vinylcyclohexene diepoxide (VCD) developed for rats (See box). These contraceptives prevent fertilization (in the case of Nicarbazin) or development of germ cells (in the case of VCD), and have only recently become available as commercial products. Products like these open up the potential for large-scale application of wildlife contraceptives, given the abundance of pigeons and rats in the world, and the potential for use in other related species.
Future research
Although scientists have conducted wildlife contraception research for decades, the focus of this research has largely not been on the welfare impacts of contraception, most likely because the primary goal has been to reduce population sizes of pest or managed species, rather than to improve welfare. Consequently, more research is needed to address the many unanswered questions regarding the possible connection between contraception and welfare. Examples of such questions include: can we expect better or worse welfare outcomes in certain taxa? If there is strong interspecific competition, will contraception lead to compensatory population growth and resource use by competitor species, eliminating the potential positive effects?
In addition to ecological considerations of contraceptive use, each of the methods requires additional scrutiny. How do different delivery methods, such as oral baits, injections and surgical sterilizations differ in their welfare effects? What are the welfare related side-effects, and in what contexts do they outweigh the possible positive welfare benefits? What are the long-term welfare effects to the treated animals? What are the indirect effects from changes in population sizes on the welfare of individuals belonging to other species?
Finally, the inter-individual dynamics of different animal populations generate additional research questions. For example, do the effects of contraception on welfare differ between solitary and social species? One reason we might expect different welfare outcomes in social and solitary species is that for social species, fewer offspring could affect the collective gathering of resources or protection from outside threats. A smaller group could be suboptimal relative to a larger social group, and contraception could therefore reduce the group size below its optimum and have negative welfare implications. Conversely, individuals of solitary species might mostly experience competitive interactions with conspecifics, where a smaller population size would be more likely to have positive welfare effects through reduced competition.
We outline a few potential projects that could help answer some of the questions regarding the welfare aspects of wildlife contraception:
Project idea 1: Empirically test the welfare effects of a contraceptive agent, with a focus on both the direct and indirect effects on individuals within the population. See an example for such a project on pigeons. Another target could be rodents (using, for example, the product ContraPest) or other species that have high fertility (with potential to improve the welfare of many individuals).
Project idea 2: Empirically or theoretically test whether and how sociality interacts with the potential welfare effects of contraception.
Project idea 3: Empirically test whether different delivery methods have different average welfare outcomes, and whether it is possible to predict which method will suit different types of species.
Project idea 4: Empirically explore the factors that determine perceptions of wildlife contraceptives, and determine public support for making improvements beyond the ‘natural’ welfare-level as opposed to just minimizing harms.
For all of the possible projects mentioned above, studying both adults and juveniles would be highly valuable when possible, given that we expect somewhat different mechanisms to mediate the welfare effects in adults compared to the welfare effects on juveniles. For instance, we would expect any reduction in sibling competition to primarily affect juvenile welfare.
Box 1
When looking for ways to test hypotheses about the welfare effects of contraceptives on wild animals, we want to look for projects where there is a potential for improvement to a lot of individuals. Rats (Rattus spp.) seem highly promising in that regard, given that they have high fertility rates. The reproductive strategy of rats results in a large number of ‘excess’ individuals who will most likely die before they reproduce. By reducing competition, contraception might increase the fraction of juveniles surviving to reproductive maturity, even if there is no change in the size of the adult population. Humans are also already exploring contraceptive interventions for them. ContraPest (developed by SenesTech) is a type of contraceptive that can be used for rats and other rodents in the United States. Testing the welfare effects of ContraPest on rodents, or indeed trying to further cut costs or develop additional types of contraceptives for rodents, seem like very promising avenues for further research.
Pigeons (Columba livia) are another promising target species for studying the welfare effects of wildlife contraceptives, as they are also very common, and there is a commercially available contraceptive for them (OvoControl, produced by Innolytics). The fertility rate of pigeons is lower than the fertility rate of rats, which therefore likely affects the potential for juvenile welfare improvement. Nevertheless, the available contraceptive for pigeons appears to be somewhat cheaper than for rats, as is indicated by my rough cost estimate for applying OvoControl and ContraPest to populations of pigeons and rats, respectively. In addition, public support for pigeon welfare (and other bird species) improvements is likely to be greater than for rats (Jaeger & Wilks 2021), so studying the welfare effects of OvoControl might have greater potential for widespread implementation. Hence, studying the welfare effects of pigeon contraception might be the most promising option, in order to eventually achieve welfare improvements for wild animals through contraception.
While currently available data does not clearly demonstrate welfare improvements from contraceptive applications, there are good theoretical reasons to expect that contraception could be extremely effective under the right conditions. Therefore, if we explicitly try to use contraception to improve wild animal welfare, it has the potential to improve the living conditions of many wild animals, especially in populations where resource scarcity is pervasive. Wildlife contraception holds great potential to improve wild animal welfare, and research to address the key theoretical and empirical questions should be a priority.
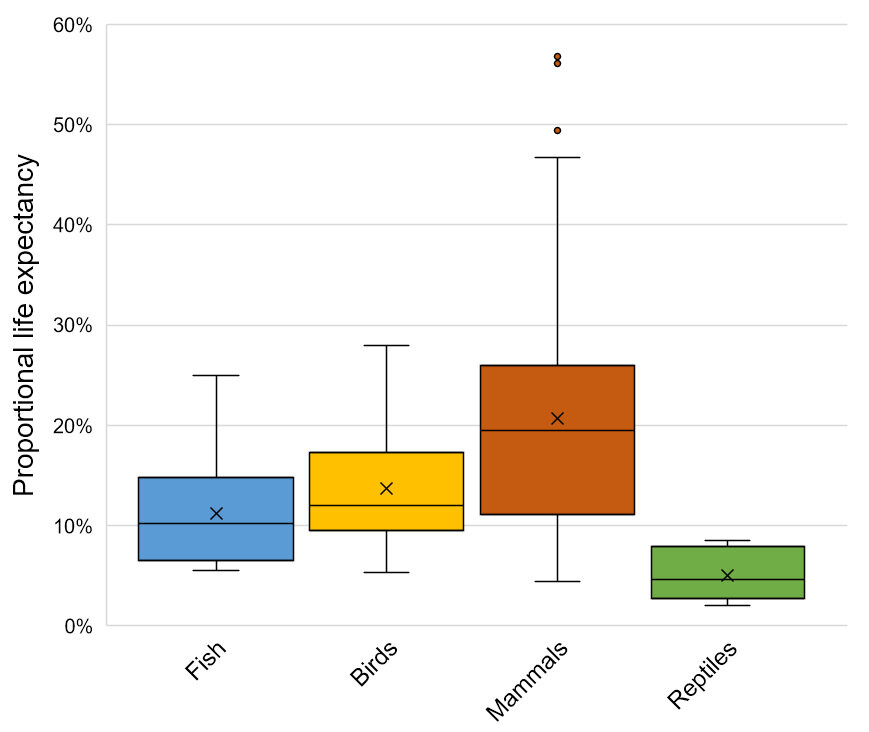
The importance of considering age when quantifying wild animals’ welfare
Hecht, L. (2021). The importance of considering age when quantifying wild animals’ welfare. Biological Reviews, https://doi.org/10.1111/brv.12769
Authored by Wild Animal Initiative’s Science Director, Luke Hecht, this paper was published in the December 2021 issue of Biological Reviews.
Abstract
Wild animals experience different challenges and opportunities as they mature, and this variety of experiences can lead to different levels of welfare characterizing the day-to-day lives of individuals of different ages. At the same time, most wild animals who are born do not survive to adulthood. Individuals who die as juveniles do not simply experience a homogeneous fraction of the lifetimes of older members of their species; rather, their truncated lives may be characterized by very different levels of welfare. Here, I propose the concept of welfare expectancy as a framework for quantifying wild animal welfare at a population level, given individual-level data on average welfare with respect to age. This concept fits conveniently alongside methods of analysis already used in population ecology, such as demographic sensitivity analysis, and is applicable to evaluating the welfare consequences of human interventions and natural pressures that disproportionately affect individuals of different ages. In order to understand better and improve the state of wild animal welfare, more attention should be directed towards young animals and the particular challenges they face.
Early-life experiences are a priority in wild animal welfare research
Hecht, L. (2021). The importance of considering age when quantifying wild animals’ welfare. Biological Reviews, 96(6), 2602-2616.
Luke Hecht explains why Wild Animal Initiative’s first call for grant proposals is focused on the welfare of juvenile animals.
Written by: Luke Hecht
Published: August 3, 2021
DOI: https://doi.org/10.71441/9nkx-wgxq
Suggested citation: Hecht, L. (August 2021), Early-life experiences are a priority in wild animal welfare research, Wild Animal Initiative, retrieved [date], https://doi.org/10.71441/9nkx-wgxq
Wild Animal Initiative recently announced our first public call for proposals, which focuses on the welfare and ecology of juvenile wild animals. Holding calls for proposals on specific themes allows us to showcase the pillars and breadth of wild animal welfare research. Here, I will explain why we think it is right to prioritize the interests of juvenile animals in most cases, drawing on my recent paper in Biological Reviews.
In many species, a majority of individuals die before reaching adulthood. For the typical wild animal, the welfare they could expect as an adult is irrelevant — but all animals experience at least some of what life is like as a juvenile. If our moral priority is making sure that as many animals as possible experience good welfare, then our practical priority should be ensuring the well-being of juvenile wild animals.
In my paper (and earlier blog post) on the importance of considering age when quantifying wild animals’ welfare, this thinking is formalized with the concept of “welfare expectancy.” Welfare expectancy represents the expected sum of welfare that a typical newborn individual will experience over their lifetime, accounting in theory for the distribution of possible welfare and lifespan outcomes. Depending on how welfare and survival rates vary with age in a given species, welfare expectancy may be especially responsive to changes in welfare and/or survival rate during particular periods of life (“welfare elasticity”, a form of proportional sensitivity analysis; Manlik et al. 2017).
If we assume there is no age-specific variation in survival rates or welfare, then lifetime welfare expectancy will always be most responsive to interventions benefiting the youngest individuals, since they will be the most numerous age group. In reality, survival rate and welfare likely do vary with age in nearly all species. It is often juveniles and senescent (very old) adults who are most vulnerable to disease, starvation and predation. Therefore, it is plausible that individuals in these demographics experience lower welfare on average than prime-age adults.
When might early-life experiences be less important to an animal’s expected lifetime welfare? In species with low juvenile survivorship and long adult life expectancies, the amount of time lived as an adult by the minority of individuals who survive their early years could exceed the amount of time lived by all the many individuals who died as juveniles. In this scenario, our priorities would likely be split between efforts to improve juvenile survival (so that a greater proportion of individuals reach adulthood) and efforts to improve adult welfare.
Species that meet these conditions tend to be less numerous, however, and are therefore unlikely to be among our priorities in the near future. For example, even North American black bears — potentially long-lived animals — appear to collectively live a majority (~55%) of their years as juveniles (based on baseline vital rates from Lewis et al. 2014). For each study or intervention, it will ultimately be important to define their target demographics and estimate how many animal life-years could potentially be improved in quality.
The everyday experiences of juvenile wild animals are little understood, yet could be highly impactful for wild animal welfare. We are excited to review your proposals for research on this important and neglected topic.
See here for more details.
Biotelemetry provides insight into a wild eagle’s death
Jane Capozzelli
Jane Capozzelli reviews how biologgers can provide information about welfare, but also pose risks to animals, following the case of a single eagle.
Written by: Jane Capozzelli
Published: December 17, 2020
DOI: https://doi.org/10.71441/zs02-dvqk
Suggested citation: Capozzelli, J. (December 2020), Biotelemetry provides insight into a wild eagle’s death, Wild Animal Initiative, retrieved [date], https://doi.org/10.71441/zs02-dvqk
Key takeaways
- Biotelemetry can provide a high level of detail about wild animals’ activities.
- Real-time behavioral and physiological data can indicate how an animal might be feeling.
- Location data can link welfare indicators to the animal’s environmental context.
- Although biotelemetry could advance efforts to improve wild animal welfare, its methods can be harmful or lethal to the animals being studied.
- Wild Animal Initiative will continue to evaluate the costs and benefits of biotelemetry. We hope to identify opportunities to collect highly detailed individual-level data without burdening the animals involved.
Introduction
Biotelemetry devices (“biologgers”) can monitor behavioral and physiological indicators of welfare status in real time, and couple these data with precise information about environmental context (Brown et al. 2013). Biologgers consist of a suite of sensors attached to an animal. The different sensors typically record location, motion, or other biological and environmental features multiple times a day (Cooke et al. 2004). The data collection is remote, meaning that the human observer does not operate in the field except to capture the animals and attach the devices.
In one such study, ornithologists outfitted a female white-tailed sea eagle (Haliaeetus albicilla) with a biologger that recorded her movement, circadian rhythm, behavioral profile, and body temperature (Krone et al. 2009). In this article, I use this case study to illustrate biotelemetry’s potential to offer insights on wild animals’ welfare.
The biologger in this case comprised two radio transmitters, a battery pack, and sensors monitoring location, temperature, vertical motion, and horizontal motion. (Figure 1). At 170 g, the device weighed 3.5% as much as the eagle. The study ended after only five months when the eagle unexpectedly died.

B.

Figure 1
A) The biologger attached to the white-tailed sea eagle (Haliaeetus albicilla). Adapted from Krone et al. (2009). B) An eagle outfitted with a biologger comparable to the “backpack” style used in the sea eagle case study. Photo credit: Alan Schumacher/USDA Wildlife Services.
As with many biotelemetry studies, this one focused on a rare species (the white-tailed sea eagle) and collected data to inform its conservation. Although welfare was not the focus of the project, it nonetheless proved data highly relevant to individual welfare because it documented the eagle’s dying process in unprecedented detail. This illustrates how biologgers could be used to understand the welfare burdens of different causes of death.
Welfare-relevant conclusions from biotelemetry
Cause of death
GPS data enabled the researchers to identify both the immediate cause of death and the broader environmental context for it.
Because the biologger transmitted location data every 5 minutes, the researchers were able to determine the precise time and location of the eagle’s death and to recover her corpse as soon as possible — overcoming major challenges in cause-of-death research. Through necropsy, they determined she died of lead poisoning (Krone et al. 2009).
Because the data stream was continuous for 5 months, there was strong evidence that the eagle had ingested lead while foraging within her territory. Even though the eagle had a high-quality habitat that was protected in a conservation reserve, she was still at risk of lead exposure because of legal hunting in her environment (Krone et al. 2009).
Welfare while dying
The biologger fitted to this eagle also included accelerometers, which provided a high-resolution behavioral profile. Activity readings were taken 8 times per second for the entire duration of the study. This frequency enabled researchers to characterize the eagle’s usual circadian rhythm, as well as the start of her sickness period and her dying period. The sickness and dying periods each lasted 11 days (Krone et al. 2009).
With an understanding of the animal’s typical daily rhythms, it was clear when she started showing abnormal behaviors that indicated disease: changes to the timing of flight and rest and increases in the frequency of rest periods (Veissier et al. 1989, Scheibe et al. 1999). The eagle eventually ceased all activity. This extreme lethargy signalled the beginning of her dying process (Krone et al. 2009). Her body temperature also dropped a total of 15℃, coinciding with the decreases in activity (Krone et al. 2009). This physiological and behavioral profile provides a basis for inferring welfare status during the dying process (Figure 2).

Figure 2
Using biotelemetry data to estimate welfare. The activity and body temperature timelines approximate the actual data Krone et al. (2009) collected from a biologger attached to a white-tailed sea eagle (Haliaeetus albicilla) in the weeks preceding her death. The inferred feelings timeline estimates the subjective mental states the eagle may have been experiencing at the same time.
I compared the eagle’s behavioural and physiological profiles over time to her baseline readings in order to estimate her mental state (Beausoleil et al. 2018). Although it may be hard to ever know with certainty how physiological conditions relate to subjective feelings in other species, we can make reasonable guesses given similarities between our own and other animals’ needs.
The eagle’s lethargy could be associated with negative feelings of malaise. The sickness and dying phases spanned 22 days, so the eagle would have also had increasing feelings of hunger, as she was not eating during this time (Mellor and Beausoliel 2015). The necropsy showed total depletion of fat reserves (Krone et al. 2009). Since eagles eagles ideally eat about 5% of their body weight daily to maintain peak body condition, (Fevold and Craighead 1958), it is reasonable to assume that this loss of reserves and lack of hunting activity would lead to feelings of severe hunger and discomfort. Lastly, the 15º C drop in body temperature would likely lead to extreme discomfort associated with coldness (Mellor and Beausoliel 2015).
Advantages of biologgers for wild animal welfare studies
Recovering corpses
For studying death in the wild, the traditional methods such as short-duration video or corpse-recovery surveys have well-known limitations. Corpses are encountered by chance (Dell et al. 2014). Most are not recovered because they are decomposed or scavenged, making it incredibly difficult to estimate the relative frequencies of different causes of death in a population (Tavecchia et al. 2011).
Biotelemetry has major advantages for cause-of-death research because it provides detailed information on when and where animals die (Klaassen et al. 2014). As a result, most of the deaths can be attributed to a specific cause (Figure 3). The positional data can also help researchers rapidly and systematically recover corpses (Calvete et al. 2002).

Figure 3
Causes of death in eagles who died during biotelemetry studies. Data from a cause-specific mortality database (Hill et al. 2019a).
Knowing the prevalence and severity of different causes of death is important, because the amount of suffering that an animal experiences while dying is largely determined by the cause and manner of death (Beausoleil et al. 2016). Vehicle collisions, hunting with firearms, or electrocution may cause near-instantaneous death, whereas disease, starvation, or intoxication may weaken the animal over an extended period (22 days in our eagle case study).
Beyond simply identifying the cause of death, comparative studies could allow us to infer the experiences of animals that die from different causes, and how the prevalence of these causes varies by life stage or environment. Such knowledge would allow wild animal welfare advocates to prioritize preventing the most painful manners of death.
Observing behavior
Currently, most welfare assessments are done by in-person human observation (Bailey et al. 2018). But biotelemetry allows continuous data collection from almost any location an animal may occupy — even where people cannot follow (Ropert-Coudert and Wilson 2005).
The ability to record quantitative behavioral profiles anywhere the animal goes allows for much more informed welfare estimates. For example, behavioral profiles can show sickness behaviors or other abnormal trends in function and condition (Brown et al. 2013, Adelman et al. 2014). This multidimensional information allows us to understand an animal’s welfare status in the context of events and environmental variables that matter at the scale of an individual’s preferences and perceptions (Bisson et al. 2009, Martins et al. 2014, McClune et al. 2015, Clemente al. 2016).
Observing community interactions
Because biologgers minimally distort behavior and can transmit data from any location, they are particularly well-suited to observing individuals’ interactions with their communities. For example, animals in different environments could be monitored as they cope with the various habitats that they encounter. This analysis would provide an animal’s-eye view on habitat quality and show to what extent an animal’s welfare might be improved or diminished by altering the environment (Lima and Zollner 1996, Nicol et al. 2020).
By pairing biotelemetry with an understanding of community interactions, it may be possible to show how community structure degrades or enhances the average welfare of each animal community-member. For example, in a community with two species that cooperate beneficially, biotelemetry could monitor the degree to which stress is reduced when animals have the opportunity to interact (Hammers et al. 2019).
Concerns about biotelemetry research
Technological limitations
Cost is the largest barrier to scaling wildlife biotelemetry research. Devices typically range from hundreds to thousands of dollars per unit (Cooke et al. 2004). Because of the high cost, sample sizes of biotelemetry studies are typically insufficient to predict a welfare outcome before it occurs, even though the data itself is reflective of welfare status (Lindberg and Walker 2007, Costantini and Møller 2013, Naef-Daenzer and Grüebler 2014).
Additionally, while biologgers have been shrinking in size due to advances in battery technology, they are still most commonly only usable on larger animals (Cooke et al. 2004). Unless the weight barriers can be overcome, the vast majority of animals — small invertebrates — are impossible to study with biotelemetry.
Among marine invertebrates, biotelemetry is generally only feasible for individuals weighing more than 400 g. If this threshold were decreased to 40 g, then ten times more species could be studied, as could larvae and juveniles (O’Dor 2002). In terrestrial insects, biotelemetry studies have been limited to a few large arthropods, such as locusts or hawkmoths. Insect biologgers usually weigh less than 1 g, but their battery life is 3 hours or less (Sato and Maharbiz 2010). This temporal limitation nearly eliminates the ability to research invertebrate welfare through biologgers.
Welfare harms
Biologgers harm the animals wearing them (Hawkins 2004). Death during the tagging process can occur either at capture, directly after release, or from physical attrition over the course of the study (Casas et al. 2015, Weiser et al. 2016, Lameris et al. 2018). For example, the eagle in our case study was caught with a noose, a particularly stressful and risky method for capturing birds (Kautz and Seamans et al. 1980).
Even in cases with no tag-related mortality (for example, Goldsmith et al. 2017), the devices can have sublethal impacts such as exhaustion, injury, reduced body condition, and the disruption of social activities and pair bonding (Weiser et al. 2016, Brlík et al. 2020, Hamelin and James 2018, Lameris et al. 2018; but see Niles et al. 2010 which found no sublethal impacts).
The eagle in our case study appeared to have no negative symptoms from the biologger (Krone et al. 2009). However, the authors noted several instances where the bird was at rest during abnormal periods of the day. The authors theorized that stresses such as hunters, tourists, inclement weather, or even sub-lethal lead exposures, may have disrupted the eagle (Krone et al. 2009). But back-mounted biologgers such as the one worn by this eagle (Figure 4) have been documented to cause significant drag that impairs flight (Irvine et al. 2007, Meyer et al. 2015). Therefore, the device itself could have periodically exhausted the bird and necessitated abnormal rest periods.
Conclusions
Biotelemetry’s scientific applications are promising for wild animal welfare research. For example, biologgers can be used to understand the prevalence of different causes of death. Biologgers provide behavioral and physiological profiles at such an extraordinary level of detail that they can potentially be used to infer animals’ mental states. As this study illustrates, these details can be discovered not only throughout an animal’s life, but uniquely, during their sickness and dying process, potentially revealing important information about the harms associated with different causes of death. Finally, by linking physiological and behavioral data with precise location data, biotelemetry gives unprecedented insight into the links between animals and their environment in real time.
The shift from direct human observation to remote monitoring is not without costs to the animals themselves. Biologgers’ weight and drag is substantial, and there are many documented instances of welfare harms and tag-related deaths. Therefore, it remains unclear whether biologgers in their current forms are justifiable or necessary for conducting wild animal welfare research. We plan to conduct further work to identify the costs and benefits associated with biologgers, best practices for determining when such work is necessary (if ever), and what can be done (if anything) to eliminate welfare harms during remote monitoring studies.
Improving pest management for wild insect welfare
Hollis Howe
Why should you care about insect welfare? Hollis Howe summarizes the literature on invertebrate sentience, estimates the number of insects affected by agricultural insecticide use, and describes the effects of common insecticidal compounds and other pest control methods.
Written by: Hollis Howe
Published: December 1, 2019
DOI: https://doi.org/10.71441/d4n3-hnty
Suggested citation: Howe H. (December 2021), Improving pest management for wild insect welfare, Wild Animal Initiative, retrieved [date], https://doi.org/10.71441/d4n3-hnty
Why insecticides?
Every year, approximately 100 million acres of American farmland are treated with lethal insecticides, killing or harming an estimated 3.5 quadrillion insects—according to our new report.
It’s currently uncertain whether insects can feel pain at all. But the sheer number killed by insecticides—and the potentially excruciating nature of those deaths—means that making pesticides more humane has a good chance of preventing large amounts of wild animal suffering.
Pesticide reform also has the advantage of being relatively easy to implement responsibly.
One of the biggest challenges to most wild animal welfare interventions is figuring out the net effect on all the species in the community. This is especially true when population sizes change. For example, falling wolf numbers might lead to rising coyote numbers, leading to falling fox numbers, leading to rising mouse numbers, leading to rising tick numbers—or they might not.
If we can replace one insecticide with another that kills the same number of insects less painfully, we can reduce suffering without changing the total insect population. That significantly narrows the range of possible unintended consequences.
The goal of this report is to lay a foundation for future projects to improve wild insect welfare by promoting more humane insect pest management practices.
Key takeaways
The evidence supporting insect sentience is sufficient to argue that, in combination with the large number of insects, we should afford some consideration to insect welfare.
Agricultural pest insect management practices may be a particularly tractable avenue for improving the expected welfare of a large number of insects.
At this point, key unknowns make it difficult to recommend a particular insecticide or non-insecticidal pest control method as more humane. However, nerve and muscle agents (such as organophosphates and carbamates, or pyrethroids and pyrethrins) are faster-acting than insecticides with other modes of action (such as insect growth regulators, or Bt toxins).
Non-insecticidal methods of pest control may be gaining popularity and should be considered when developing more humane pest insect management practices. The net welfare impact of non-lethal methods is dependent on ecological factors and the specific organisms involved.
Next steps
In addition to the report itself, I developed two tools to facilitate further research.
The first is a database of insecticidal compounds, their modes of action, and their insecticidal mechanisms. This database is under development and may be expanded to include pests targeted, brand names, and chemical fact sheets where available.
I have also developed a rough impact estimate table outlining a method for using a pest control literature review to calculate the minimum number of insects affected by U.S. agricultural insecticide use.
As part of our upcoming strategic planning and inter-organization coordination process, Wild Animal Initiative will be evaluating the course of our future research on insect welfare. Currently, it looks like the most promising direction would be to fill in the gaps necessary to evaluate specific insecticide interventions:
What are the economic injury levels (EILs) for particular crop-pest pairs?
Can EILs be used to generate estimates of the number of insects affected by pest management?
On which insect species and crops are particular insecticides or pest management methods used?
In the process of creating this report, many more questions arose than were answered. Despite this, the report provides an overview of the insect welfare landscape and the potential avenues for further intervention research.
How wild animals die: what we know so far
In part three of this series, Luke Hecht gives an overview of what research in this field has taught us so far about how wild animals die and highlights gaps that seem especially important for welfare biology.
Written by: Luke Hecht
Published: December 1, 2020
DOI: https://doi.org/10.71441/8s71-bpfd
Suggested citation: Hecht, L. (December 2020), How wild animals die: what we know so far, Wild Animal Initiative, retrieved [date], https://doi.org/10.71441/8s71-bpfd
This is the final post in three-part series. Read part one → Read part two →
Key takeaways
- Many studies have investigated the causes of death of adult mammals and birds, but there is a lack of data on the deaths of juvenile animals, as well as fish and insects.
- Large, adult animals are more likely to be killed by humans than by any other cause. Small-bodied animals and juveniles are more susceptible to predation by other wild animals.
- Technological improvements in underwater monitoring will improve our understanding of wild fish mortality.
- Insects often die in very different ways from larger animals. Most of what we know about insects’ deaths comes from agricultural pest control research.
- Populations need to be studied under a range of conditions to predict how their cause-specific mortality rates might change under different scenarios.
Introduction
Previously, I wrote about approaches to studying wild animals’ causes of death, with the goal of making work in this field maximally useful for understanding wild animal welfare. Cause of death has not received sufficient research attention relative to its significance to wild animal welfare. The overwhelming majority of the research that does exist is focused on land-dwelling mammals and birds, and primarily on cases where understanding what animals are dying from is instrumental to preventing the extinction of their species. Here, I will give an overview of what research in this field has taught us so far about how wild animals die and highlight gaps that seem especially important for welfare biology.
Cause of death in wild terrestrial vertebrates
Collins and Kays (2011) conducted the first systematic review of cause-specific mortality rates in mammals, selecting only studies that used small radio trackers to monitor animals and document their deaths in a timely manner. They also limited their analysis to adult animals, due to a serious lack of data on juvenile mortality causes. This review found that, overall, predation was the most common cause of death for small-bodied mammals, while human-caused deaths, including hunting and vehicle collision, were the most common causes of death in larger mammals.
In 2019, Hill et al. expanded on this review with a vast amount of new data, including data from juveniles and non-mammalian vertebrates. They found that natural causes of death (predation, disease, starvation), especially predation, were common among juvenile animals irrespective of their species’ typical adult body size. For mammals and reptiles, predation was roughly twice as frequent in juveniles as in adults, with predation accounting for more than 95% of documented deaths of juvenile reptiles (Figure 1). Birds, on the other hand, exhibited very similar cause-specific mortality rates between juvenile and adult age classes.
The authors note that their dataset probably still greatly underestimates natural causes of death, considering that these deaths are often harder to detect. Although smaller animals exhibit the highest natural mortality rates, they are harder to monitor. Research to date has also focused on populations that come into conflict with or are used by humans. These factors combine to make the most numerous deaths in the wild the least understood.

Figure 1
Cause-specific mortality rates separated by age and taxonomic class. Adapted from Hill et al. (2019).
Hill et al. provide an excellent summary of mortality causes, but many of these categories — especially predation, accident and disease — encompass a huge degree of variation in experience. For example, 18% of New Zealand sea lions die from tuberculosis, while 24% die as a result of violence from other sea lions (Lenting et al. 2019). Similarly, approximately half of American black bears die as cubs, often as a result of attacks by older male bears (LeCount 1987). In their study area of northern Pennsylvania, Alt (1984) estimated that 5% of cubs drowned in their winter dens as a result of flash flooding. This was 3-5 times more likely to occur when dens were made in root cavities or excavated soil. Juvenile bears also frequently die from disease (e.g. Chomel et al. 1998). For example, a grizzly cub suffering from canine hepatitis died live on camera in Katmai National Park beside their bewildered mother and siblings. Welfare interventions will need to account for all this variation in individual wild animals’ experiences of death.
Much of the data on birds’ deaths comes from urban wildlife hospitals, where traumatic injury is often the apparent leading cause of death among adult birds, while disease and malnutrition is responsible for the majority of juvenile deaths (e.g. Stenkat et al. 2013). However, these statistics are certain to be biased by the kinds of birds and causes of death that people are most likely to come into contact with. For example, although many traumatic injuries are attributable to domestic cats, birds attacked by predatory raptors are rarely admitted (c.f. Palma et al. 2006). Studies of radio-tagged wild birds report predation and hunting as the leading causes of death (Hill et al. 2019). Some disease mortality can also be linked to hunting practices, as the importation of birds to stock hunting grounds can contribute to outbreaks (Buenestado 2009). Migratory birds present a special challenge for researchers, since many of their deaths occur in transit over long distances, where it may be impossible to recover their corpses. For example, out of 51 probable deaths among satellite-tracked migratory raptors, Klaasen et al. (2014) were only able to confirm 10, among which the leading causes of death were collision with man-made structures and exhaustion during migration.
The progress made in the last decade of cause-specific mortality research is encouraging, but there is much more to be learned, especially about more numerous, smaller-bodied animals. Little is known about the causes of death among prairie dogs, for instance, except that predation is common and plague occasionally wipes out entire colonies (Crosby and Graham 1986; Stapp et al. 2004). Studies of wild reptiles, especially snakes and turtles, consistently indicate road traffic accidents as a leading cause of death (e.g. Himes et al. 2002), although most juveniles are killed by predators (e.g. Butler and Sowell 1996). These gaps in our knowledge of wild animal mortality seem likely to be filled as radio tracking devices are made smaller, lighter and more resilient over the next decade (Kays et al. 2015).
Cause of death in wild fish
Among vertebrates, the most neglected group in the current body of cause-specific mortality research is wild fish. Of the papers that do touch on causes of death in wild fish, many are case reports focusing on the risks of disease outbreaks to human health or the fishing industry. Case reports can be valuable for demonstrating the presence of specific risks that some number of fish do die from (Krkošek 2017). For example, Sterud et al. (2007) detected an outbreak of the parasite Tetracapsuloides bryosalmonae in an Atlantic salmon population that appeared to be their most common cause of death during the study period. This parasite is probably in constant circulation among the fish, and outbreaks have been documented in many other populations, but it is impossible to say whether these results are a representative snapshot of the parasite’s effect on mortality or what alternative causes of death occur in the population.
Most effective studies of terrestrial animals’ cause of death rely on recovering the corpses of tagged individuals, as discussed in my previous post. However, this is clearly more challenging to do with fish. Radio signals for tracking individuals are relatively ineffective underwater, and corpses are much less likely to be recovered (c.f. Benelli and Pozzebon 2013). Several studies have cleverly taken advantage of avian predators naturally retrieving radio-tagged fish from the water and depositing their eaten corpses on shore. For example, Koed et al. (2006) estimated that 39% and 12% of juvenile salmon and brown trout, respectively, were preyed upon by cormorants in the estuary where their study was set. Approximately a further 10% of salmon and trout were killed by cormorants in the adjoining river, while 3-6% were preyed upon by pike, a predatory fish. A similar study by Dieperink et al. (2001) recorded that 65% of juvenile trout were eaten by cormorants and herons while migrating downstream to the Baltic Sea. Fritts and Pearsons (2004) used another predator-based approach to quantify predation, analyzing the stomach contents of smallmouth bass to estimate their annual consumption of salmon. Taken at face value, their results suggested that smallmouth bass accounted for only around 4% of salmon mortality in this system, though the authors noted that this was probably an underestimate. Despite the special challenges involved in recovering the corpses of wild fish, it is at least possible to study the impact of specific predators on fish populations.
Technological progress is proving especially important for studying cause of death in the marine environment. In one recent study, fish were implanted with acoustic transmitters that enabled the researchers to detect predation events occurring within range of their underwater receiver array (Weinz et al. 2020). They inferred that approximately one third of tagged fish were preyed upon over the four months following their release. Several factors could be confounding this result, in both directions. For one, the invasive procedure required to implant the fish with acoustic transmitters could have made these particular fish more susceptible to predation than average. This would be consistent with other studies showing that fish caught and released are more vulnerable due to physical injuries or stress (e.g. Raby et al. 2013). On the other hand, some predation events were likely missed because they occurred out of range of the acoustic array. As the range of underwater tracking improves and the size of transmitting devices decreases so as to cause less harm to the tagged individuals, we should expect to see much more data on the lives and deaths of fish and other marine animals.
Cause of death in insects
Insects are among the most numerous and diverse animals, but also the smallest. Their diversity and size makes them especially difficult to monitor on an individual level. At the same time, some insect species are viewed as pests, and so there is commercial interest in understanding their natural causes of death to devise more effective management strategies (Roux and Baumgärtner 1998). This has motivated a handful of studies reviewing the causes of death of agriculturally relevant insects (Table 1).
Some of these studies use a combination of field observations and population modeling to estimate by how much the population’s overall mortality rate would be reduced if a given cause of death could be eliminated. For example, if predators disproportionately target weak or sick prey, then even if many individuals ultimately die from predation, eliminating their predators may have only a small effect on the overall mortality rate because the same individuals who are vulnerable to predation are also vulnerable to dying soon from disease or starvation.

Table 1
Summarized results from eight studies on cause-specific mortality in six insect species from a pest management context.
Determining the significance of competing mortality risks
Future interventions seeking to improve wild animal welfare should account for how actions to protect animals from specific causes of death could influence population sizes, lifespans, and alternative causes of death. Compensatory mortality, where mortality due to a certain cause is replaced by mortality due to another cause when the first is removed, has been noted in many diverse species and ecosystems. For example, Hostetter et al. (2012) reported that cormorants along the Columbia River in Oregon selectively predate poorer-condition juvenile steelhead salmon en route to the sea. Had they not been killed by cormorants, these same individuals would likely have been among the first to succumb to harsh conditions and competition during their first winter in the ocean (Hurst 2007). Similarly, in a classic study on mule deer, Bartmann et al. (1992) found that removal of coyotes over winter reduced the rate of mule deer deaths by predation, but did not increase their overall survival rates, as the researchers observed increased mortality due to starvation. Diseased mule deer are also selectively preyed upon by mountain lions (e.g. Krumm et al. 2009), which suggests that some of this predator-caused mortality is compensatory.
On the other hand, some cause-specific mortality really is irreplaceable. Achhami et al. (2020) demonstrated this for plant chemical defenses against wheat stem sawflies, while Cooley et al. (2009) showed that other causes of death are not reduced in populations of mountain lions hunted by humans. Bergman et al. (2015) proposed that the degree to which mortality due to a particular cause is compensatory depends on ecological context. For example, if chronic illness made animals less effective at competing for food in a dense population, death by starvation may occur first and compensate for some disease-related mortality in this hypothetical population. If food later became more accessible, we might observe an apparent increase in mortality caused by diseases that are now able to run their course. In addition to studying mortality dynamics in a snapshot of a population’s present conditions, research should model how mortality risks compete under different conditions (Siler 1979) (Figure 2).

Figure 2
Three hypothetical scenarios for the dynamics of mortality due to predation on a prey population. In all scenarios, baseline mortality in the absence of predators is 50 individuals per year. If predator-induced mortality is completely additive (purple diamonds), 10 more animals dying to predation increases total mortality by 10. If predation is completely compensatory (blue squares), total mortality stays constant despite an increased number of deaths by predation; instead, there are commensurately fewer deaths by other causes. In most cases, mortality due to a given cause is likely to be partially compensatory (green circles), lying somewhere between these two extremes. In this model, low rates of predation are mostly compensatory, perhaps removing old or sick individuals from the population. As the intensity of predation increases, a larger proportion of healthy animals are killed and so predation mortality becomes increasingly additive.
Conclusions
A relatively comprehensive snapshot of cause of death in the wild is emerging for terrestrial mammals, birds, and reptiles, thanks to mainstream research in ecology and conservation (Hill et al. 2019). For example, we can see that predation is the leading natural cause of death, especially in juveniles (Figure 1). However, this account is still likely to be heavily biased, since most studies focus on adults and large-bodied species. The focus on large animals could be inflating the number of deaths caused by hunting rather than vehicle collisions, for instance, given that accidents involving small animals often go unreported (Sáenz-de-Santa-María and Tellería 2015). More research on juveniles and small mammals is needed (Table 2). We are also at the stage of considering interventions to improve the welfare of some wild mammals and birds, ranging from pigeon fertility control to reducing feral cat predation. To make these interventions as effective as possible, it would be valuable to understand the replaceability of different sources of mortality.

Table 2
Research gaps and proposed priorities by taxon.
Fish and juvenile amphibians are still seriously lacking in data on cause-specific mortality, especially relating to juveniles. Research on both of these groups is challenged by the fact that they spend some or all of their lives underwater, but biologging technologies — including pop-up satellite archival tags (PSATs) (Tolentino et al. 2017) and acoustic telemetry (Weinz et al. 2020) — could enable more research on cause of death in aquatic environments, especially related to predation.
A surprising amount is known about cause-specific mortality in insects regarded as agricultural pests (Table 1). However, very little research has been done on the deaths of insects with less agricultural relevance. From the existing research, it is clear that insects face challenges that would surprise us. For example, Asiimwe et al. (2006) found that many whiteflies died as a result of poorly understood developmental abnormalities, such as failure to complete metamorphosis. Drowning during rainy weather was one of the foremost causes of death among the moths of Pereira et al. (2007), and plant chemical defenses were responsible for a majority of the deaths of wheat stem sawflies monitored by Acchami et al. (2020).
As many challenges as we know that animals face in the wild, some may yet be masked by the urgent threats they face from humans through hunting, fishing, extermination, and competition for resources. Humans should leave more space for wildlife, and try not to make their existence harder than it already is, but we should also be willing to go further. Learning more about cause-specific mortality in different populations and environmental contexts can inform plans for the most effective actions to improve wild animal welfare.
References
Achhami, Buddhi B., Robert K. D. Peterson, Jamie D. Sherman, Gadi V. P. Reddy, and David K. Weaver. 2020. ‘Multiple Decrement Life Tables of Cephus Cinctus Norton (Hymenoptera: Cephidae) across a Set of Barley Cultivars: The Importance of Plant Defense versus Cannibalism’. PLOS ONE 15 (9): e0238527. https://doi.org/10.1371/journal.pone.0238527.
Andis, M. D., and C. L. Meek. 1985. ‘Mortality and Survival Patterns for the Immature Stages of Psorophora Columbiae’. Journal of the American Mosquito Control Association 1 (3): 357–62.
Asiimwe, P., J. S. Ecaat, M. Otim, D. Gerling, S. Kyamanywa, and J. P. Legg. 2007. ‘Life-Table Analysis of Mortality Factors Affecting Populations of Bemisia Tabaci on Cassava in Uganda’. Entomologia Experimentalis et Applicata 122 (1): 37–44. https://doi.org/10.1111/j.1570-7458.2006.00487.x.
Bartmann, Richard M., Gary C. White, and Len H. Carpenter. 1992. ‘Compensatory Mortality in a Colorado Mule Deer Population’. Wildlife Monographs, no. 121: 3–39.
Benelli, G., Pozzebon, A., & Reaz, M. B. I. 2013. ‘RFID under water: Technical issues and applications’ (pp. 379-395). Rijeka: InTech. https://doi.org/10.5772/53934.
Bergman, Eric J., Paul F. Doherty, Gary C. White, and A. Andrew Holland. 2015. ‘Density Dependence in Mule Deer: A Review of Evidence’. Wildlife Biology 21 (1): 18–29. https://doi.org/10.2981/wlb.00012.
Buenestado, F. J., Ferreras, P., BLANCO‐AGUIAR, J. A., Tortosa, F. S., & Villafuerte, R. 2009. ‘Survival and causes of mortality among wild Red‐legged Partridges Alectoris rufa in southern Spain: implications for conservation’. Ibis 151 (4): 720-730. https://doi.org/10.1111/j.1474-919X.2009.00952.x.
Butler, J. A., & Sowell, S. 1996. ‘Survivorship and predation of hatchling and yearling gopher tortoises, Gopherus polyphemus’. Journal of Herpetology 30 (3): 455-458. https://doi.org/10.2307/1565195.
Chomel, B.B., R.W. Kasten, G. Chappuis, M. Soulier, and Y. Kikuchi. 1998. ‘Serological Survey of Selected Canine Viral Pathogens and Zoonoses in Grizzly Bears (Ursus Arctos Horribilis) and Black Bears (Ursus Americanus) from Alaska: -EN- -FR- -ES-’. Revue Scientifique et Technique de l’OIE 17 (3): 756–66. https://doi.org/10.20506/rst.17.3.1134.
Collins, C., and R. Kays. 2011. ‘Causes of Mortality in North American Populations of Large and Medium-Sized Mammals’. Animal Conservation 14 (5): 474–83. https://doi.org/10.1111/j.1469-1795.2011.00458.x.
Cooley, Hilary S., Robert B. Wielgus, Gary M. Koehler, Hugh S. Robinson, and Benjamin T. Maletzke. 2009. ‘Does Hunting Regulate Cougar Populations? A Test of the Compensatory Mortality Hypothesis’. Ecology 90 (10): 2913–21. https://doi.org/10.1890/08-1805.1.
Crosby, Lyle A., and Randy Graham. 1986. ‘Population Dynamics and Expansion Rates of Black-Tailed Prairie Dogs’. Proceedings of the Vertebrate Pest Conference 12 (12). https://escholarship.org/uc/item/23t218h1.
Dieperink, C., S. Pedersen, and M. I. Pedersen. 2001. ‘Estuarine Predation on Radiotagged Wild and Domesticated Sea Trout (Salmo Trutta L.) Smolts’. Ecology of Freshwater Fish 10 (3): 177–83. https://doi.org/10.1034/j.1600-0633.2001.100307.x.
Fritts, Anthony L., and Todd N. Pearsons. 2004. ‘Smallmouth Bass Predation on Hatchery and Wild Salmonids in the Yakima River, Washington’. Transactions of the American Fisheries Society 133 (4): 880–95. https://doi.org/10.1577/T03-003.1.
Hill, Jacob E., Travis L. DeVault, and Jerrold L. Belant. 2019. ‘Cause-Specific Mortality of the World’s Terrestrial Vertebrates’. Global Ecology and Biogeography 28 (5): 680–89. https://doi.org/10.1111/geb.12881.
Himes, J. G., Hardy, L. M., Rudolph, D. C., & Burgdorf, S. J. 2002. ‘Growth rates and mortality of the Louisiana pine snake (Pituophis ruthveni)’. Journal of Herpetology 36 (4): 683-687. https://doi.org/10.2307/1565941.
Hostetter, Nathan J., Allen F. Evans, Daniel D. Roby, and Ken Collis. 2012. ‘Susceptibility of Juvenile Steelhead to Avian Predation: The Influence of Individual Fish Characteristics and River Conditions’. Transactions of the American Fisheries Society 141 (6): 1586–99. https://doi.org/10.1080/00028487.2012.716011.
Hurst, T. P. 2007. ‘Causes and Consequences of Winter Mortality in Fishes’. Journal of Fish Biology 71 (2): 315–45. https://doi.org/10.1111/j.1095-8649.2007.01596.x.
Jones, Davy. 1982. ‘Predators and Parasites of Temporary Row Crop Pests: Agents of Irreplaceable Mortality or Scavengers Acting Prior to Other Mortality Factors?’ Entomophaga 27 (3): 245–65. https://doi.org/10.1007/BF02374809.
Karut, K., and S. E. Naranjo. 2009. ‘Mortality Factors Affecting Bemisia Tabaci Populations on Cotton in Turkey’. Journal of Applied Entomology 133 (5): 367–74. https://doi.org/10.1111/j.1439-0418.2008.01369.x.
Kays, Roland, Margaret C. Crofoot, Walter Jetz, and Martin Wikelski. 2015. ‘Terrestrial Animal Tracking as an Eye on Life and Planet’. Science 348 (6240). https://doi.org/10.1126/science.aaa2478.
Klaassen, R. H., Hake, M., Strandberg, R., Koks, B. J., Trierweiler, C., Exo, K. M., ... & Alerstam, T. 2014. ‘When and where does mortality occur in migratory birds? Direct evidence from long‐term satellite tracking of raptors’. Journal of Animal Ecology 83 (1): 176-184. https://doi.org/10.1111/1365-2656.12135.
Koed, Anders, Henrik Baktoft, and Brian Daniel Bak. 2006. ‘Causes of Mortality of Atlantic Salmon (Salmo Salar) and Brown Trout (Salmo Trutta) Smolts in a Restored River and Its Estuary’. River Research and Applications 22 (1): 69–78. https://doi.org/10.1002/rra.894.
Krkošek, Martin. 2017. ‘Population Biology of Infectious Diseases Shared by Wild and Farmed Fish1’. Canadian Journal of Fisheries and Aquatic Sciences, January. https://doi.org/10.1139/cjfas-2016-0379.
Krumm, Caroline E., Mary M. Conner, N. Thompson Hobbs, Don O. Hunter, and Michael W. Miller. 2010. ‘Mountain Lions Prey Selectively on Prion-Infected Mule Deer’. Biology Letters 6 (2): 209–11. https://doi.org/10.1098/rsbl.2009.0742.
LeCount, Albert L. 1987. ‘Causes of Black Bear Cub Mortality’. Bears: Their Biology and Management 7: 75–82. https://doi.org/10.2307/3872610.
Lenting, B., B. Gartrell, A. Kokosinska, P. J. Duignan, S. Michael, S. Hunter, and W. D. Roe. 2019. ‘Causes of Adult Mortality in Two Populations of New Zealand Sea Lions (Phocarctos Hookeri)’. Veterinary and Animal Science 7 (June): 100057. https://doi.org/10.1016/j.vas.2019.100057.
Naranjo, Steven E., and Peter C. Ellsworth. 2005. ‘Mortality Dynamics and Population Regulation in Bemisia Tabaci’. Entomologia Experimentalis et Applicata 116 (2): 93–108. https://doi.org/10.1111/j.1570-7458.2005.00297.x.
Palma, L., Beja, P., Pais, M., & Cancela Da Fonseca, L. 2006. ‘Why do raptors take domestic prey? The case of Bonelli's eagles and pigeons’. Journal of Applied Ecology 43 (6): 1075-1086. https://doi.org/10.1111/j.1365-2664.2006.01213.x.
Pereira, E. J. G., M. C. Picanço, L. Bacci, A. L. B. Crespo, and R. N. C. Guedes. 2007. ‘Seasonal Mortality Factors of the Coffee Leafminer, Leucoptera Coffeella’. Bulletin of Entomological Research 97 (4): 421–32. https://doi.org/10.1017/S0007485307005202.
Peterson, Robert K. D., Ryan S. Davis, Leon G. Higley, and Odair A. Fernandes. 2009. ‘Mortality Risk in Insects’. Environmental Entomology 38 (1): 2–10. https://doi.org/10.1603/022.038.0102.
Raby, Graham D., Jessica R. Packer, Andy J. Danylchuk, and Steven J. Cooke. 2014. ‘The Understudied and Underappreciated Role of Predation in the Mortality of Fish Released from Fishing Gears’. Fish and Fisheries 15 (3): 489–505. https://doi.org/10.1111/faf.12033.
Roux, Olivier, and Johann Baumgärtner. 1998. ‘Evaluation of Mortality Factors and Risk Analysis for the Design of an Integrated Pest Management System’. Ecological Modelling 109 (1): 61–75. https://doi.org/10.1016/S0304-3800(98)00035-0.
Sáenz-de-Santa-María, A., & Tellería, J. L. 2015. ‘Wildlife-vehicle collisions in Spain’. European Journal of Wildlife Research 61 (3): 399-406. https://doi.org/10.1007/s10344-015-0907-7.
Siler, W. 1979. ‘A competing‐risk model for animal mortality’. Ecology 60 (4): 750-757. https://doi.org/10.2307/1936612.
Stapp, Paul, Michael F. Antolin, and Mark Ball. 2004. ‘Patterns of Extinction in Prairie Dog Metapopulations: Plague Outbreaks Follow El Ninì Events’. Frontiers in Ecology and the Environment 2 (5): 235–40. https://doi.org/10.1890/1540-9295(2004)002[0235:POEIPD]2.0.CO;2.
Stenkat, J., Krautwald-Junghanns, M. E., & Schmidt, V. 2013. ‘Causes of morbidity and mortality in free-living birds in an urban environment in Germany’. Ecohealth 10 (4): 352-365. https://doi.org/10.1007/s10393-013-0868-9.
Sterud, E, T Forseth, O Ugedal, Tt Poppe, A Jørgensen, T Bruheim, H Fjeldstad, and Ta Mo. 2007. ‘Severe Mortality in Wild Atlantic Salmon Salmo Salar Due to Proliferative Kidney Disease (PKD) Caused by Tetracapsuloides Bryosalmonae (Myxozoa)’. Diseases of Aquatic Organisms 77 (October): 191–98. https://doi.org/10.3354/dao01846.
Tolentino, Emily R., Russell P. Howey, Lucy A. Howey, Lance K. B. Jordan, R. Dean Grubbs, Annabelle Brooks, Sean Williams, Edward J. Brooks, and Oliver N. Shipley. 2017. ‘Was My Science Project Eaten? A Novel Approach to Validate Consumption of Marine Biologging Instruments’. Animal Biotelemetry 5 (1): 3. https://doi.org/10.1186/s40317-016-0117-4.
Weinz, Amy A., Jordan K. Matley, Natalie V. Klinard, Aaron T. Fisk, and Scott F. Colborne. 2020a. ‘Identification of Predation Events in Wild Fish Using Novel Acoustic Transmitters’. Animal Biotelemetry 8 (1): 28. https://doi.org/10.1186/s40317-020-00215-x.
Methods for studying wild animals’ causes of death
In part two of this series, Luke Hecht introduces some of the tools and approaches used to study wild animals’ causes of death.
Written by: Luke Hecht
Published: November 2, 2020
DOI: https://doi.org/10.71441/1fat-f9e3
Suggested citation: Hecht, L. (November 2020), Methods for studying wild animals’ causes of death, Wild Animal Initiative, retrieved [date], https://doi.org/10.71441/1fat-f9e3
Key takeaways
- Cause of death is an important area of wild animal welfare research.
- Accurate cause-specific mortality rates are difficult to obtain.
- Multistate modeling can provide insight where information about cause-specific mortality rates is incomplete.
- Experimental studies and improved monitoring technology can improve our ability to diagnose cause of death, estimate cause-specific mortality rates, and understand compensatory mortality.
- Future research should identify and focus on the causes of death that involve the most suffering.
In a world with perfect monitoring of wild populations, biologists could be immediately alerted to the death of an animal, show up on the scene, and perform a necropsy to determine the animal’s precise cause of death. They would also know everything about the age and health history of this animal, having monitored them from birth. Unfortunately, it is not practical to monitor most wild populations at nearly this level of detail. Our understanding of how and why wild animals die must be pieced together from statistical models and sparse empirical data. In this post, I will introduce some of the tools and approaches used to study wild animals’ causes of death.
Death assemblages
The most straightforward way to study wild animals’ causes of death is to scour a habitat for corpses. The resulting collection is sometimes called a “death assemblage.” When the body of a recently deceased animal is recovered, indicators such as injuries, signs of disease, and stomach contents may make it possible to determine what killed them (Behrensmeyer and Miller 2012).
Determining cause of death from serendipitously recovered corpses depends on finding animals soon after their deaths. Death assemblage surveys are therefore most useful for animals whose remains are more likely to be discovered, like coastal marine mammals. Their large size means they take a while to decay, and their bodies often wash up on beaches where they are highly visible and quickly reported to authorities or researchers (Tinker et al. 2016). Recovery of well-preserved corpses is much less common for animals who are small or live in lush environments like forests, which typically provide more concealment and support more rapid decomposition.
The probability of recovering a corpse can also be biased by whatever caused the animal’s death (Gerber et al. 2004). For example, the bodies of animals who were killed by predators may be less likely to be recovered because they are almost entirely consumed on the spot or are carried away from the site (e.g. hyenas stashing bones of prey, Kuhn 2005). On the other hand, animals who die from starvation, disease, old age, or something else that leads to their gradual weakening may attempt to conceal themselves in their final hours. While their bodies are likely to remain intact for longer, they may go unnoticed by researchers.
Certain causes of death are also inherently more difficult to diagnose, so researchers need to be wary of simply ignoring data in an “unknown” category that may be disproportionately filled with instances of hard-to-distinguish causes of death. For example, in a survey of bones found in a hyena den, presumably belonging to their past prey, Kuhn (2005) identified roughly a quarter of the specimens to species level. Without further information, any quantitative claims about the composition of the local ecosystem based on these data would have to be taken with serious caution because the remaining 75% of “unknown” specimens might be drawn unequally from underlying populations. For example, animals who are morphologically similar to other species, or animals who are not preferred prey for hyenas, might be underrepresented in resulting estimates.
Bias is a pervasive problem in cause of death surveys. A naive survey of rabbit corpses in a particular forest might conclude that most recent deaths were attributable to some rapidly debilitating disease, while in fact the majority have been carried off by birds of prey without a trace. To estimate the true rates of alternative causes of death from corpse recovery data, researchers need to account for the fact that the forensic clues to certain causes of death may be more or less detectable than others, depending on a host of circumstances.
Accounting for incomplete information
The inevitable incompleteness of corpse recovery surveys can be partially offset by explicitly modeling sources of uncertainty along the chain of events from death by a specific cause, to corpse recovery, to cause identification. This approach is sometimes called multistate modeling (Hougaard 1999; Gauthier and Lebreton 2009). These models make a distinction between the objective chain of events that occurred to an individual animal and the observable evidence of those events (Koons et al. 2014). For example, researchers generally do not directly observe rodents being preyed on by raptors. A fraction of predation events leave behind bits of fur or the partially eaten body of the rodent. Researchers then have a particular probability of encountering these bits of evidence and deducing the correct cause of death.
Box 1
To illustrate what a multistate model looks like in practice, imagine that a flock of birds begins to roost every day in a tree outside where you live. The foliage is dense, so you cannot see all of the roosting birds at any one time through your window. One of the birds (“Bird X”) has distinctive markings, with which you can usually distinguish them from the others. One day, you can’t see Bird X in the tree. Your first worry is that Bird X might have died, but then you remember that they might just be out of view behind a branch. A week passes and Bird X still hasn’t been seen in the tree. Is it more likely that you simply have not caught sight of Bird X for seven days in a row, or that Bird X died sometime in the week?
This problem can be solved by estimating the exact proportion of birds in the tree who can be seen on any given day (“detection probability”), the probability of recognizing the identity of Bird X if you set eyes on them (“identification probability”), and the average daily survival probability for each bird in this population. These can then be incorporated into a model where Bird X is considered to be in one of two states: alive or dead. Using the model, we can determine whether our series of observations of the tree outside is better explained by Bird X being alive or by being dead. Marescot et al. (2015) applied a similar sort of model to observations of tagged black-tailed deer to estimate their age-specific survival rates and the impact of predation on their population growth.
While multistate modeling is frequently used to estimate survival rates in wild animal populations, similarly nuanced studies of cause of death are rare. This may reflect both a general disinterest in cause-of-death research and a greater confidence in the raw data that does exist. Studying human-caused deaths or species for which there is a good infrastructure for monitoring removes some uncertainty and lessens the need for multistate models. These statistical methods are most important to apply when studying populations where the vast majority of individuals’ deaths go undocumented, leaving room for large biases in the data. For example, Gerber et al. (2004) investigated cause-specific mortality of sea otters. To do so, they took advantage of an extensive network of marine mammal research groups to recover and analyze carcasses that washed up on the beach. Using a demographic model, they estimated that their sample represented just under half of all mortality during the study period. To sample half of all deceased individuals in a wild population of marine mammals is a remarkable achievement, but it highlights how much potential there is for unequal cause-specific recovery rates to produce biased results, especially in less intensively monitored populations. In marine mammals, for example, different predator species may hunt at different distances from shore, or near rocky cliffs as opposed to smooth beaches. Either of those factors could affect the probability of a carcass washing up onshore and being recovered by researchers (Joly et al. 2009).
The biggest weakness of purely observational studies of wild animal mortality is that it is difficult to know what data are missing. The simplest way of overcoming this is to tag a set number of animals at the beginning of a study and only count the remains of tagged individuals. This sort of study design is still subject to biased recovery rates, but the magnitude of bias is limited because corpses of untagged individuals are excluded. Schaub and Pradel (2004) used such an approach to estimate the proportion of white storks killed in airborne collisions with power lines in Switzerland (Figure 1a). They found that simply counting corpses overestimated the proportion of juvenile white storks killed by power lines by about 25%. It makes sense that the bodies of birds killed by power line collisions would be more conspicuous than birds who die otherwise, because the ground around power lines tends to be more exposed to allow for maintenance access. Power lines may also serve as natural concentrating points for associated deaths, whereas deaths by other causes may be uniformly distributed across the landscape.
Limiting analyses to tagged animals is effective at preventing overestimation of particular causes of death, but it can also leave researchers with a lot of missing data from individuals whose bodies were not recovered or who lost their tags. Steps can be taken to improve the recovery rate of tagged animals, such as attaching radio trackers to animals alongside conventional tags (Tavecchia et al. 2011; Figure 1b). Records of when a tagged animal was last seen alive can also complement corpse recovery data, because they allow for independent estimation of survival rates and rates of tag loss. Without this information, corpse recovery data alone cannot distinguish between an animal who is still alive and one who died but was never recovered. Fortunately, technical improvements in the precision and durability of radio trackers are making it practical to do this remotely. Even with relatively basic GPS tags, statistical models can distinguish tag loss or technical failure from actual death of the tagged animal with almost perfect accuracy (Sergio et al. 2018). Electronic tags capable of recording animals’ fine-scale movements or vital signs can also provide evidence related to an animal’s cause of death, such as how sudden it was or what posture the animal adopted in their final moments (Cooke 2008; Brown et al. 2013). Advances in the field of animal remote sensing will be helpful for learning about cause-specific mortality at a level of detail that is currently impossible for researchers to observe.

Figure 1
Multistate models treat wild animals’ fates — observed or unobserved — as the product of a series of conditional probabilities reflecting the combination of events over their life. A) A basic model that considers whether an animal is alive or dead, what caused their death, and whether their corpse was recovered (Schaud and Pradel 2004). B) A more complex model that estimates the probabilities of a bird dying from poisoning or electrocution, and ultimately the probability of a given bird being sighted again alive or dead (Tavecchia et al. 2011). In both of these models, the state transition probabilities are estimated based on the history of encounters between researchers and each tagged animal over the course of a study. For example, if researchers observe an individual after not sighting them for multiple field seasons, then that sighting might both increase the estimated survival rate and decrease the estimated detection rate.
Experimental study of cause-specific mortality
Rather than simply observing the fates of wild animals, experimental approaches directly exclude or introduce specific causes of death. The most common form of this approach is to exclude predators with fencing or other deterrents. For example, Smith et al. (2012) excluded all medium-sized mammalian predators from some nesting grounds of the gopher tortoise, but not others, and found that tortoises born in the predator exclusion zones were almost twice as likely to still be alive one year later. Similarly, Conner et al. (2011) found that exclusion fencing reduced the number of cotton rats killed by mammals. However, increased predation by birds and snakes largely made up for the decreased predation by mammals, especially after a forest fire destroyed much of the undergrowth that could otherwise conceal the rats.
Although most experimental research on cause-specific mortality to date has focused on hunting or predation, a similar approach could be used to study the effectiveness of interventions against other common causes of death, including curable diseases or hunger (c.f. Rickett et al. 2013). However, interventions like supplemental feeding, disease prevention and predator exclusion may result in compensatory mortality, where individuals spared from one cause of death are at elevated risk of dying soon after because of another cause. The most effective way of learning about compensatory mortality risk is to use an experimental rather than observational study design, in conjunction with the same best practices for observational studies as described previously (Bergman et al. 2015).
Outlook for welfare-focused cause of death research
From the perspective of wild animal welfare, understanding cause-specific mortality is morally urgent. A few shifts in the current approach to cause-of-death research will help us determine which issues to prioritize to help animals. Most importantly, much more research should be focused on the most common deaths in the wild: deaths of juvenile animals and members of species with large global populations. Because they are so numerous, these individuals’ experiences of dying are especially important to understand if we value all sentient animals’ welfare equally. These animals tend to be more difficult to monitor due to their smaller body size, but studying their mortality is becoming ever easier with new technology. At the current rate of progress, organisms weighing around one gram could be tracked via GPS within a decade or less (Kays et al. 2015).
One major source of uncertainty in wild animal mortality research is the diagnosis of an animal’s cause of death. This is especially true for low-tech studies, where corpses are encountered by chance, rather than sought at a particular location based on radio tracking and a mortality signal. As a result, corpses may be significantly decomposed or scavenged. Even under ideal circumstances, it can be difficult to determine the order in which apparent injuries occurred. In lieu of perfect monitoring, researchers could perform detailed analysis on a subset of the recovered bodies to evaluate the reliability of their field diagnoses of the rest, as Joly et al. (2009) did with sea otter carcasses. Studies could also be dedicated to intensive monitoring of a relatively small number of animals so that when they die the cause can be known with high confidence and researchers can quickly find their corpses to document field-identifiable characteristics and how these change over the coming days or weeks, as well as estimate recovery rates. The field of taphonomy has long filled a similar supportive role for paleobiology (Behrensmeyer and Miller 2012). Upfront investment in this sort of meta-research could enable subsequent studies of wild animal mortality to accomplish more with less (c.f. Loannidis 2018).
Welfare-focused researchers should aim to identify and focus on the causes of death that entail much more suffering than others. This could involve a reframing of cause-specific mortality research where rather than asking how animals of a given species are likely to die, researchers ask who the victims of a particular cause of death are likely to be. Rather than tracking marmots and determining what proportion were preyed on by cougars, for instance, researchers could monitor cougars using cameras or fecal analysis to learn who their prey were (Popanom et al. 2011). This sort of cause-centric approach might also be appropriate in contexts where a single ultimate cause can lead to several different proximate causes of death which vary in their severity. For example, researchers could survey a forest in the aftermath of a fire and try to determine what proportion of fatalities occurred by burning, asphyxiation, subsequent starvation, or other proximate causes related to the fire (Gutiérrez and Javier de Miguel, 2020). Simply verifying that a certain cause of death occurred at least once in a given population may be useful to inform future work. If so, low-cost cause-specific mortality studies could use a form of rarefaction analysis to judge how comprehensively the causes of death present in a population have been sampled, with a focus on enumerating the causes rather than estimating their relative frequencies.
Efforts to improve the health and lifespans of our fellow humans are supported by publicly available data on the most common causes of death. This is part of how governments decide which public health interventions and fields of medical research to allocate funding to. A comparable wealth of data could help us identify which wild animals need the most urgent aid, and what form that aid should take. In the next part of this series, I will give an overview of some of the data that does exist on the causes of wild animal mortality.
References
Behrensmeyer, Anna K., and Joshua H. Miller. 2012. ‘Building Links Between Ecology and Paleontology Using Taphonomic Studies of Recent Vertebrate Communities’. In Paleontology in Ecology and Conservation, edited by Julien Louys, 69–91. Springer Earth System Sciences. Berlin, Heidelberg: Springer. https://doi.org/10.1007/978-3-642-25038-5_5.
Bergman, Eric J., Paul F. Doherty, Gary C. White, and A. Andrew Holland. 2015. ‘Density Dependence in Mule Deer: A Review of Evidence’. Wildlife Biology 21 (1): 18–29. https://doi.org/10.2981/wlb.00012.
Brown, Danielle D., Roland Kays, Martin Wikelski, Rory Wilson, and A. Peter Klimley. 2013. ‘Observing the Unwatchable through Acceleration Logging of Animal Behavior’. Animal Biotelemetry 1 (1): 20. https://doi.org/10.1186/2050-3385-1-20.
Conner, L. Mike, Steven B. Castleberry, and Anna M. Derrick. 2011. ‘Effects of Mesopredators and Prescribed Fire on Hispid Cotton Rat Survival and Cause-Specific Mortality’. The Journal of Wildlife Management 75 (4): 938–44. https://doi.org/10.1002/jwmg.110.
Cooke, Steven J. 2008. ‘Biotelemetry and Biologging in Endangered Species Research and Animal Conservation: Relevance to Regional, National, and IUCN Red List Threat Assessments’. Endangered Species Research 4 (1–2): 165–85. https://doi.org/10.3354/esr00063.
Gauthier, Gilles, and Jean-Dominique Lebreton. 2008. ‘Analysis of Band-Recovery Data in a Multistate Capture-Recapture Framework’. Canadian Journal of Statistics 36 (1): 59–73. https://doi.org/10.1002/cjs.5550360107.
Gerber, Leah R., M. Tim Tinker, Daniel F. Doak, James A. Estes, and David A. Jessup. 2004. ‘Mortality Sensitivity in Life-Stage Simulation Analysis: A Case Study of Southern Sea Otters’. Ecological Applications 14 (5): 1554–65. https://doi.org/10.1890/03-5006.
Gutiérrez, J. and de Miguel, F. J. 2020. Challenges posed by fires to wild animals and how to help: A literature review, Oakland: Animal Ethics, retrieved from https://www.animalethics.org/fire-wild-animals-help.
Hougaard, Philip. 1999. ‘Multi-State Models: A Review’. Lifetime Data Analysis 5 (3): 239–64. https://doi.org/10.1023/A:1009672031531.
Ioannidis, John P. A. 2018. ‘Meta-Research: Why Research on Research Matters’. PLOS Biology 16 (3): e2005468. https://doi.org/10.1371/journal.pbio.2005468.
Joly, Damien O., Dennis M. Heisey, Michael D. Samuel, Christine A. Ribic, Nancy J. Thomas, Scott D. Wright, and Irene E. Wright. 2009. ‘Estimating Cause-Specific Mortality Rates Using Recovered Carcasses’. Journal of Wildlife Diseases 45 (1): 122–27. https://doi.org/10.7589/0090-3558-45.1.122.
Kays, R., M. C. Crofoot, W. Jetz, and M. Wikelski. 2015. ‘Terrestrial Animal Tracking as an Eye on Life and Planet’. Science 348 (6240): aaa2478–aaa2478. https://doi.org/10.1126/science.aaa2478.
Koons, David N., Marlène Gamelon, Jean-Michel Gaillard, Lise M. Aubry, Robert F. Rockwell, François Klein, Rémi Choquet, and Olivier Gimenez. 2014. ‘Methods for Studying Cause-Specific Senescence in the Wild’. Methods in Ecology and Evolution 5 (9): 924–33. https://doi.org/10.1111/2041-210X.12239.
Kuhn, Brian. 2005. ‘The Faunal Assemblages and Taphonomic Signatures of Five Striped Hyaena (Hyaena Hyaena Syriaca) Dens in the Desert of Eastern Jordan’. Levant 37 (1): 221–34. https://doi.org/10.1179/lev.2005.37.1.221.
Marescot, Lucile, Tavis D. Forrester, David S. Casady, and Heiko U. Wittmer. 2015. ‘Using Multistate Capture–Mark–Recapture Models to Quantify Effects of Predation on Age-Specific Survival and Population Growth in Black-Tailed Deer’. Population Ecology 57 (1): 185–97. https://doi.org/10.1007/s10144-014-0456-z.
Pompanon, Francois, Bruce E. Deagle, William O. C. Symondson, David S. Brown, Simon N. Jarman, and Pierre Taberlet. 2012. ‘Who Is Eating What: Diet Assessment Using Next Generation Sequencing’. Molecular Ecology 21 (8): 1931–50. https://doi.org/10.1111/j.1365-294X.2011.05403.x.
Rickett, Jennifer, Cody J. Dey, Jillian Stothart, Constance M. O’Connor, James S. Quinn, and Weihong Ji. 2013. ‘The Influence of Supplemental Feeding on Survival, Dispersal and Competition in Translocated Brown Teal, or Pateke (Anas Chlorotis)’. Emu - Austral Ornithology 113 (1): 62–68. https://doi.org/10.1071/MU12053.
Schaub, Michael, and Roger Pradel. 2004. ‘Assessing the Relative Importance of Different Sources of Mortality from Recoveries of Marked Animals’. Ecology 85 (4): 930–38. https://doi.org/10.1890/03-0012.
Sergio, Fabrizio, Alessandro Tanferna, Julio Blas, Guillermo Blanco, and Fernando Hiraldo. 2019. ‘Reliable Methods for Identifying Animal Deaths in GPS- and Satellite-Tracking Data: Review, Testing, and Calibration’. Journal of Applied Ecology 56 (3): 562–72. https://doi.org/10.1111/1365-2664.13294.
Smith, Lora L., David A. Steen, L. Mike Conner, and Jessica C. Rutledge. 2013. ‘Effects of Predator Exclusion on Nest and Hatchling Survival in the Gopher Tortoise’. The Journal of Wildlife Management 77 (2): 352–58. https://doi.org/10.1002/jwmg.449.
Tavecchia, Giacomo, Jaume Adrover, Antoni Muñoz Navarro, and Roger Pradel. 2012. ‘Modelling Mortality Causes in Longitudinal Data in the Presence of Tag Loss: Application to Raptor Poisoning and Electrocution’. Journal of Applied Ecology 49 (1): 297–305. https://doi.org/10.1111/j.1365-2664.2011.02074.x.
Tinker, M. Tim, Brian B. Hatfield, Michael D. Harris, and Jack A. Ames. 2016. ‘Dramatic Increase in Sea Otter Mortality from White Sharks in California’. Marine Mammal Science 32 (1): 309–26. https://doi.org/10.1111/mms.12261.
Reducing the burden of disease: the One Health approach
Jane Capozzelli
Jane Capozzelli reviews the One Health approach to public health and veterinary medicine, identifying research themes that could be prioritized to most improve wild animal welfare.
Written by: Jane Capozzelli
Published on: October 5, 2020
DOI: https://doi.org/10.71441/08qc-343t
Suggested citation: Capozzelli, J. (October 2020), Reducing the burden of disease: the One Health approach, Wild Animal Initiative, retrieved [date], https://doi.org/10.71441/08qc-343t
Abstract
I review the One Health approach to public health and veterinary medicine and present three contemporary research projects.
One Health is motivated by the maxim that the health and well-being of domestic animals, people, and wildlife are inextricably linked. Many projects focus on mitigating wildlife diseases in ways that help humans or livestock.
These projects can benefit the welfare of wild animals by improving the target animals’ health. To maximize wild animal welfare benefits, One Health projects should prioritize targeting common species and particularly prolonged or painful health issues.
Key takeaways
Although One Health does not optimize for wild animal welfare, it represents a tractable opportunity to expand existing efforts in a beneficial direction.
One Health researchers seem to care about wildlife health for the sake of wild animals’ welfare, not just because of the ways wildlife health issues affect people.
Demonstrating that improving wild animal welfare can also benefit public health or livestock health might appeal to people who do not typically prioritize animal welfare.
Established approaches for wildlife health research are relatively easy to extend to high-priority issues in wild animal welfare.
Introduction to One Health
Health, though not the only determinant of good welfare, is a key component (Mellor and Beausoleil 2015). By replacing feeling sick with feeling healthy, good health improves welfare. For the purposes of this article, when I refer to improvements to wild animal welfare, I mainly refer to improving the health of target animals.
Wildlife health projects are promising avenues for improving wild animal welfare. Many of these projects are designed using the “One Health” approach. The framework views both the human and non-human worlds as morally important and interconnected (Schwabe 1984, Zinsstag et al. 2001). For example, veterinarians swear an oath to use their knowledge for both “the promotion of public health” and “the prevention and relief of animal suffering” (Mackenzie et al. 2013). More specifically, One Health follows the maxim that the health and welfare of animals and people are inextricably linked to each other and to the quality of the environment (Osborn et al. 2009).
One Health has been highly successful relative to other health paradigms (Osofsky and Pongsiri 2018). Historically, wildlife health, livestock health, and human health were treated as separate problems. But solving today’s most complex health problems needs expertise in all three areas (Whitmee et al. 2015). Addressing wildlife health, livestock health, and human health collectively — as part of “One Health” — ultimately yields benefits that can’t be found by addressing each problem individually.
Some leading institutions in the One Health movement include One Health Commission and the One Health Initiative. The CDC also includes “One Health” as an important public health approach. Several large projects also originate from the AVMA-accredited colleges of veterinary medicine.
Existing One Health projects
Removing exclusion fencing
Veterinary cordon fences exclude wild herbivores from rangelands throughout southern Africa to reduce the transmission of foot-and-mouth disease between wild herbivores and livestock (Gadd et al. 2012). Foot-and-mouth disease is nonlethal to livestock, but has painful symptoms. Wild animals typically carry the disease asymptomatically (Young et al. 1972, Vosloo et al. 2007).
Exclusion fencing may degrade wild animals’ quality of life more than foot-and-mouth disease itself. Exclusion fencing fragments home ranges and disrupts migration routes. When their movement is constrained, animals cannot escape from potential harms as easily. As a result, cordoned animals more frequently die from starvation, dehydration, electrocution, and entanglement (Gadd et al. 2012, Lindsey et al. 2012).
The costs of exclusion fencing also permeate the socio-economic climate in Africa (ibid, ibid). Outbreaks of foot-and-mouth disease still occur due to fencing failures or mistakes in the food regulatory chain (Thomson et al. 2018). Meat from exposed livestock cannot be sold (ibid), a burden that is largest for low-income producers (Whitmee et al. 2015).
Exclusion fencing does not eradicate poverty from foot-and-mouth disease, and it creates a number of welfare problems for vast numbers of wild animals. The One Health solutions are two-fold: change the regulations for beef so that the process is more humane for people and livestock (MacKenzie et al. 2013), and dismantle fencing. Deaths by entanglement, electrocution, and exclusion-related starvation and dehydration are reduced, and restoring migratory routes improves animals' abilities to act on their behavioral preferences (Gadd et al. 2012, Mellor and Beausoleil 2015, Allen et al. 2018).
Eradicating tuberculosis
Tuberculosis, a severe bacterial infection of the lungs, circulates among human, livestock, and wildlife hosts. Many low-income countries have not controlled tuberculosis. In Tanzania, for example, as many as 16% of all people have had the disease (Travis et al. 2019), as have 1%-13% of cattle, depending on the region (Katale et al. 2013).
An active One Health project has identified buffalo (Syncerus caffer) as a major disease reservoir (Travis et al. 2019, Roug et al. 2020a) and revealed that habitat loss and intensive cattle grazing in and around natural areas have exacerbated transmission by bringing wild buffalo in more frequent contact with people and domestic animals (Kirksey 2012, Roug et al. 2020b).
Rangeland degradation appears to be an overarching problem contributing to poor health from tuberculosis. Following the One Health approach, reducing habitat loss may be a single-source solution that benefits the quality of life of many people and animals.
Surveilling emerging infectious diseases
“Emerging infectious diseases” is an umbrella term for pathogens that are newly discovered, recently evolved, or occupying new ranges or hosts (Rachowicz et al. 2005, Dazak et al. 2000, Bird and Mazet 2018). Emerging infectious diseases tolerated in one host can become epidemics in others — resulting in extinctions, suffering, or economic damage (Rachowicz et al. 2005, Barrett 2014, Zukai 2014). One Health projects to address this include the establishment of a global disease surveillance system with the capacity to respond to these health threats in real time (Bird and Mazet 2018).
This surveillance network is applied to diseases that primarily affect wild animals. Wild rhinoceroses and giraffes have recently displayed novel ulcerative skin infections. The cause in rhinos is thought to be a roundworm, but for giraffes it remains a mystery (Mutinda et al. 2012, Muneza et al. 2016). Disease monitoring efforts like these are a promising tool for learning more about diseases that primarily affect wild animals.
Uncounted numbers of diseases circulate among different wildlife species, but because of the lack of information on diseases that do not (yet) affect people, we have almost no information on the costs these unmonitored pathogens have for wild animals (Dazak et al. 2000). Understanding disease dynamics helps people stop the spread of wildlife diseases to new populations and intervene to restore good health to wild animals currently burdened by disease.
Future research to improve wild animal welfare
Target the worst diseases
One Health has been used many times to address diseases in wild animals. But in some of these instances, wild animals are merely asymptomatic carriers of these diseases. Pathogens that cause painful and prolonged symptoms in wild animals more significantly decrease their welfare (Beausoleil et al. 2012, Allen et al. 2018).
These diseases should be prioritized. Research that compares the relative severity of emerging infectious diseases — regardless of whether the diseases are transmissible to livestock or people — could be a promising way to apply One Health’s disease surveillance network to address wild animal welfare.
It is also important to increase our capacity to address wildlife health issues so that we can treat the prolonged and painful diseases once we discover them. Wildlife health facilities are also a valuable repository of information about wildlife health issues on the ground (Loyd et al. 2017). Expanding these facilities helps us understand which diseases are most common or cause the most suffering.
Target common animals
Common species such as urban animals, livestock competitors, and agricultural pests tend to be of minimal conservation concern because of their abundance. But welfare efforts targeting common animals are likely to be most cost effective per capita because interventions can be distributed to vast numbers of individuals.
Pigeons heavily populate human-animal interfaces — areas in the landscape where human and non-human animals cohabitate (Kirksey 2012, Pacini-Ketchabaw and Nxumalo 2015). Because pigeons live in cities, they are vulnerable to avoidable and devastating anthropogenic health problems (Murray et al. 2019).
Targeting common animals like pigeons has strong potential to ameliorate welfare issues due to poor health on a large scale. Citizen science data from New York City pigeons indicate that on average, 165 pigeons annually are poisoned by lead (Cai and Calisi 2016). There is no reason to expect this rate to dissipate with time. It is also a minimum estimate because the data is sourced from one wildlife rehabilitation center. If this rate is reflected in all 33 megacities, then at least 5445 pigeons are poisoned each year globally.
Conclusion
Human, animal, and environmental health are linked. It is essential to address healthcare in wild animals to ensure they have good welfare. Veterinarians and wildlife health scientists who follow the One Health approach care about wildlife health for the sake of wild animals’ welfare, not just because wildlife health issues spill over to people.
One Health continues to make strides toward building a global healthcare system for people, domestic animals, and wild animals. Though One Health projects tend to focus on implications for livestock and human health, there is potential for expansion into a range of wild animal welfare issues. A maximally beneficial welfare intervention would target common animals and their most prolonged and painful health issues.
One Health represents an unusual example of institutions and funding dedicated to improving wildlife health and engages a wide range of scientists and veterinarians. A wild animal welfare project that demonstrates co-benefits to livestock and people could similarly engage these practitioners by demonstrating that improving the welfare of wild animals is an important, multi-dimensional societal issue.
Why cause of death matters for wild animal welfare
In part one of this series, Luke Hecht introduces approaches to studying wild animals’ causes of death, with the goal of making work in this field maximally useful for understanding wild animal welfare.
Written by: Luke Hecht
Published: September 23, 2020
DOI: https://doi.org/10.71441/qcjb-e4b4
Suggested citation: Hecht, L. (September 2020), Why cause of death matters for wild animal welfare, Wild Animal Initiative, retrieved [date], https://doi.org/10.71441/qcjb-e4b4
This is the first post in a series. Read part two →
The most fundamental reason for viewing death as a bad thing is that it deprives individuals of future experiences, assuming those experiences would have been predominantly positive. However, the process of dying is also often painful in itself, and an individual’s death can have additional negative impacts—emotional and material—on others, including family and the broader population.
The costs of a death depend on its cause. Humans are willing to sacrifice periods of healthy life to avoid especially unpleasant deaths, and see various causes of death as more fearsome than others (Sunstein 1997; Chapple et al. 2006). Of course, people’s perception of how much suffering particular deaths would entail may be biased by things like squeamishness; they may assume that because a death is gruesome it must be especially painful. The terminal stages in the process of dying, during which suffering presumably outweighs pleasure, comprise a small fraction of most humans’ lives. Willingness to trade years of healthy life to obtain a quicker, less painful death implies that either the original manner of death must be extremely bad, or that we have an exaggerated sense of how bad dying is.
As recently as the 19th century, only around half of newborns could expect to survive to adulthood (Riley 2005). Many human cultures have had traditions of only conferring names on children when they reached a certain age, potentially because it was understood that most would not survive that long (but that if they did, they would have a decent prospect of reaching adulthood) (Lancy 2014). For example, birth registration was not a legal requirement in the United Kingdom until 1874 (ONS 2015). As average lifespan increases, the dying process represents an ever smaller proportion of human life.
While human childhood mortality has declined dramatically throughout much of the world, most wild animals still die at a young age relative to the longest-lived members of their own species (Figure 1). For these animals, the process of dying may actually represent a substantial portion of their lifetimes, suggesting that, in addition to knowing the most likely causes of death for entire populations, it would be especially valuable to know which manners of death are common among juveniles in specific wild animal populations.
Figure 1: A boxplot showing life expectancy as a percentage of a species’ maximum lifespan for 152 populations of fish (n=16), birds (n=54), mammals (n=72) and reptiles (n=10). Life expectancies were calculated from models found in the COMADRE database (Salguero-Gomez et al. 2016), and maximum lifespans were obtained from AnAge (De Magalhães et al. 2005). Across major vertebrate classes, most individuals live to only 10-30% of the age of the oldest known individuals of their species.
“Cause” of death can be interpreted in at least two different ways: “manner” of death, or “ultimate cause” of death. Both matter for wild animal welfare, but they can be difficult to disentangle in practice. For example, an animal who died during a forest fire may have died by burning or asphyxiation. Other animals may die in the immediate aftermath of a forest fire from dehydration or infected burn wounds. The welfare impact of these different manners of death would likely vary, and it is possible in theory to rank their welfare impacts based on the intensity and duration of suffering leading up to death (Figure 2; Sharp and Saunders 2011). Learning about the precise manner in which wild animals die can help us prioritize hazards to protect them from to reduce instances of the most extreme suffering. On the other hand, it is often easier to estimate the number of animals who died due to an ultimate cause - such as forest fires - than to break this down into specific manners of death. Learning about an ultimate cause of death, and the number of deaths resulting from it, is also useful for prioritizing welfare interventions directed at extending good lives.
Figure 2: An example scheme for grading the welfare impact of different manners of death, taking into account both the severity of suffering inflicted and the length of time it takes for the animal to die. Adapted from work by Sharp and Saunders (2011) on wild animal culling methods.
To minimize the suffering animals experience in the wild, we need to understand how and why wild animals die, paying particular attention to the most numerous experiences: that is, the deaths of juvenile animals belonging to common species. The rates of different causes of death are also valuable to know because even if dying turns out to be a minor contributor to lifetime welfare, or the difference in severity between manners of death is relatively low, substituting one manner of death for another less painful one could improve individual welfare without affecting population size, minimizing the number of variables we need to consider to determine whether an intervention is worth implementing.
In a series of posts to follow, I will review the methods available for studying causes of wild animal deaths and present a selection of published data on cause-specific mortality rates. Finally, I’ll describe some original modeling by former Wild Animal Initiative Intern Anthony DiGiovanni that explicitly considers how cause of death may change along with the size and demography of wild animal populations.
References
Sunstein, C. (1997). Bad deaths. Journal of Risk and Uncertainty, 14(3), 259-282.
Chapple, A., Ziebland, S., McPherson, A., & Herxheimer, A. (2006). What people close to death say about euthanasia and assisted suicide: a qualitative study. Journal of Medical Ethics, 32(12), 706-710.
Riley, J. C. (2005). Estimates of regional and global life expectancy, 1800–2001. Population and Development Review, 31(3), 537-543.
Lancy, D. (2014). “Babies aren’t persons”: A survey of delayed personhood. In H. Otto & H. Keller (Eds.), Different Faces of Attachment: Cultural Variations on a Universal Human Need (pp. 66-110). Cambridge: Cambridge University Press.
Office for National Statistics. (2015). How has life expectancy changed over time? Retrieved from https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/articles/howhaslifeexpectancychangedovertime/2015-09-09.
Sharp, T., & Saunders, G. (2011). A model for assessing the relative humaneness of pest animal control methods. Canberra, Australia: Department of Agriculture, Fisheries and Forestry.
De Magalhaes, J. P., & Costa, J. (2009). A database of vertebrate longevity records and their relation to other life‐history traits. Journal of Evolutionary Biology, 22(8), 1770-1774.
Salguero‐Gómez, R., Jones, O. R., Archer, C. R., Bein, C., de Buhr, H., Farack, C., ... & Römer, G. (2016). COMADRE: a global database of animal demography. Journal of Animal Ecology, 85(2), 371-384.